CV – Research – Lab members – Publications
 Prof. Dr. Maarten H. P. Kole
Prof. Dr. Maarten H. P. Kole
Cell Biology, Faculty of Science, University of Utrecht.
e-mail: M.H.P.kole@uu.nl
Netherlands Institute for Neuroscience,
Royal Netherlands Academy for Arts and Sciences (KNAW),
Meibergdreef 47, 1105 BA,
Amsterdam, The Netherlands
Ph : +31 20 566 4594
Ph : +31 20 566 4901
Fax: +31 20 566 6121
http://herseninstituut.nl/onderzoek/onderzoeksgroepen/kole-groep/
Curriculum Vitae
Maarten Kole studied Biology at the University of Groningen and during his PhD (2003), at the Leibniz Institute for Primate Research (Göttingen, Germany), specialized in electrophysiological recording of voltage-gated and synaptic ion channels. He went for his postdoctoral studies to the laboratory of Prof. Dr. Greg Stuart at the John Curtin School of Medical Research, Australian National University. Here, investigating biophysical properties of ion channels he established some of the first direct sub-cellular recordings in mammalian axons. In 2009 he became appointed as Team Leader. Maarten Kole is recipient of AW Campbell Award (2010) from the Australian Neuroscience Society and an ERC Starting Grant (2011). In 2011 he was appointed as Group Leader at the Netherlands Institute for Neuroscience, Amsterdam.
Research
Our research group focuses on the excitability and functions of mammalian axons. Emerging near the cell body of neurons, axons perform the main fundamental operations in the brain; they integrate synaptic potentials, convert these into action potential codes and conduct the output signals towards the presynaptic terminal, often millimeters away from the cell body. These critical operations have an electrical basis generated by specific repertoires of voltage-gated ion channels, clustered either to the axon initial segment or nodes of Ranvier. In order to understand their properties and roles in neural computations we use electrophysiological patch-clamp techniques in combination with live high-resolution video, confocal- or two-photon microscopy enabling the selective targeting of specific cellular domains of axons.
One of our main research topics is focused on the axonal voltage-gated ion channels. By using high temporal-resolution recording we explore their biophysical properties in the distinct domains of the axon, called the axon initial segment and the nodes of Ranvier. For example, previous work showed that sodium channels have often compartment-specific voltage-dependence of channel gating.
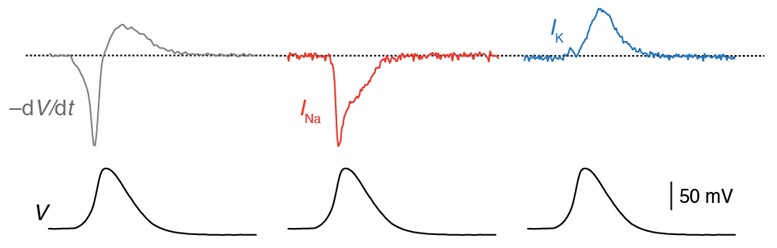
Axonal action potential voltage-clamp protocol (bottom). The voltage-clamp waveform evoked isolated Na+ current (middle) and K+ currents (right) in axonal outside-out patches. Adapted from Hallermann et al. 2012.
By employing immuno-fluorescent labeling for ion channel subtypes we aim to localize, identify and characterize the voltage-gated ion channels mediating axonal excitability. Direct electrophysiological recording configurations including whole-cell, cell-attached and outside-out patch are employed to generate novel conductance-based models and integrate these into NEURON simulation software. Computational experiments are extremely useful to analyze parameters in isolation, enable experiments that would otherwise be impossible and to explore concepts of axonal functions. Our long-term goal is to construct an empirically-constrained computational model of a cortical myelinated pyramidal neuron axon and scale the insights from nanometer to millimeter resolution.
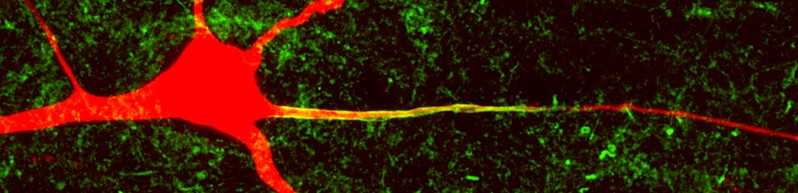
Layer 5 pyramidal neuron cell body, dendrites and axon (filled, red). Co-labeling for the voltage-gated sodium channel (Pan-Nav, green).
Another current research line focuses on the anatomical and functional organization of axonal myelination. Oligodendrocytes wrap multi-lamellar myelin layers around cortical pyramidal neuron axons. In fact, the vast majority of axons in the mammalian nervous system are myelinated. To understand their functions in the layer 5 axons we investigate how neuron-glia interactions contribute to myelination, how activity affects myelination and, vice versa, how myelin loss impacts on voltage-gated ion channels.
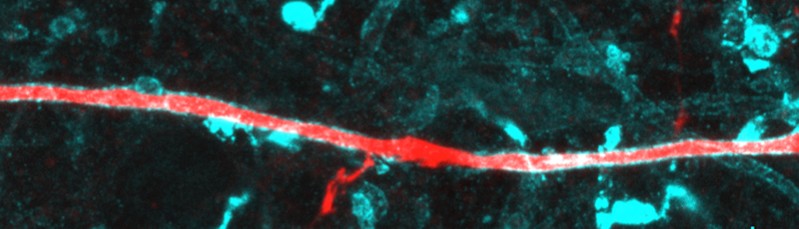
Pyramidal neuron axon (red) co-stained for myelin basic protein (MBP, cyan). The myelin gap in the middle represents a node of Ranvier with collateral.
Lab members
Technicians:
Sharon de Vries s.devries@nin.knaw.nl
Postdocs:
Marko Popovic m.popovic@nin.knaw.nl
Mohit Dubey m.dubey@nin.knaw.nl
Arne Battefeld a.battefeld@nin.knaw.nl
PhD students:
Charles Cohen c.cohen@nin.knaw.nl
M. Alfonso Malpede m.malpede@nin.knaw.nl
Naomi Hanemaaijer n.hanemaaijer@nin.knaw.nl

Publications
2024
Seignette K, Jamann N, Papale P, Terra H, Porneso RO, de Kraker L, van der Togt C, van der Aa M, Neering P, Ruimschotel E, Roelfsema PR, Montijn JS, Self MW, Kole MHP, Levelt CN. Experience shapes chandelier cell function and structure in the visual cortex. Elife. 2024 Jan 9;12:RP91153. doi: 10.7554/eLife.91153. PMID: 38192196.
2023
Wilbers R, Metodieva VD, Duverdin S, Heyer DB, Galakhova AA, Mertens EJ, Versluis TD, Baayen JC, Idema S, Noske DP, Verburg N, Willemse RB, de Witt Hamer PC, Kole MHP, de Kock CPJ, Mansvelder HD, Goriounova NA. Human voltage-gated Na+ and K+ channel properties underlie sustained fast AP signaling. Sci Adv. 2023 Oct 13;9(41):eade3300. doi: 10.1126/sciadv.ade3300. Epub 2023 Oct 12. PMID: 37824607.
Fréal A, Jamann N, Ten Bos J, Jansen J, Petersen N, Ligthart T, Hoogenraad CC, Kole MHP. Sodium channel endocytosis drives axon initial segment plasticity. Sci Adv. 2023 Sep 15;9(37):eadf3885. doi: 10.1126/sciadv.adf3885. Epub 2023 Sep 15. PMID: 37713493.
Passchier EMJ, Kerst S, Brouwers E, Hamilton EMC, Bisseling Q, Bugiani M, Waisfisz Q, Kitchen P, Unger L, Breur M, Hoogterp L, de Vries SI, Abbink TEM, Kole MHP, Leurs R, Vischer HF, Brignone MS, Ambrosini E, Feillet F, Born AP, Epstein LG, Mansvelder HD, Min R, van der Knaap MS. Aquaporin-4 and GPRC5B: old and new players in controlling brain oedema. Brain. 2023 Aug 1;146(8):3444-3454. doi: 10.1093/brain/awad146. PMID: 37143309.
van den Bosch AMR, Hümmert S, Steyer A, Ruhwedel T, Hamann J, Smolders J, Nave KA, Stadelmann C, Kole MHP, Möbius W, Huitinga I. Ultrastructural Axon- Myelin Unit Alterations in Multiple Sclerosis Correlate with Inflammation. Ann Neurol. 2023 Apr;93(4):856-870. doi: 10.1002/ana.26585. Epub 2023 Jan 5. PMID: 36565265.
2022
Kole K, Voesenek BJB, Brinia ME, Petersen N, Kole MHP. Parvalbumin basket cell myelination accumulates axonal mitochondria to internodes. Nat Commun. 2022 Dec 9;13(1):7598. doi: 10.1038/s41467-022-35350-x. PMID: 36494349; PMCID: PMC9734141.
Jamann N, Kole MHP. Connecting axons and dendrites: An oblique view. Neuron. 2022 May 4;110(9):1438-1440. doi: 10.1016/j.neuron.2022.04.014. PMID: 35512635
Dubey M, Pascual-Garcia M, Helmes K, Wever DD, Hamada MS, Kushner SA, Kole MHP. Myelination synchronizes cortical oscillations by consolidating parvalbumin-mediated phasic inhibition. Elife. 2022 Jan 10;11:e73827. doi: 10.7554/eLife.73827. PMID: 35001871
2021
Ramaglia V, Dubey M, Malpede MA, Petersen N, de Vries SI, Ahmed SM, Lee DSW, Schenk GJ, Gold SM, Huitinga I, Gommerman JL, Geurts JJG, Kole MHP. Complement- associated loss of CA2 inhibitory synapses in the demyelinated hippocampus impairs memory. Acta Neuropathol. 2021 Oct;142(4):643-667. doi: 10.1007/s00401-021-02338-8. Epub 2021 Jun 25. PMID: 34170374.
Siemons ME, Hanemaaijer NAK, Kole MHP, Kapitein LC. Robust adaptive optics for localization microscopy deep in complex tissue. Nat Commun. 2021 Jun 7;12(1):3407. doi: 10.1038/s41467-021-23647-2. PMID: 34099685; PMCID: PMC8184833.
Jamann N, Dannehl D, Lehmann N, Wagener R, Thielemann C, Schultz C, Staiger J, Kole MHP, Engelhardt M. Sensory input drives rapid homeostatic scaling of the axon initial segment in mouse barrel cortex. Nat Commun. 2021 Jan 4;12(1):23. doi: 10.1038/s41467-020-20232-x. PMID: 33397944; PMCID: PMC7782484.
2020
Moore S, Meschkat M, Ruhwedel T, Trevisiol A, Tzvetanova ID, Battefeld A, Kusch K, Kole MHP, Strenzke N, Möbius W, de Hoz L, Nave KA. A role of oligodendrocytes in information processing. Nat Commun. 2020 Oct 30;11(1):5497. doi: 10.1038/s41467-020-19152-7. PMID: 33127910; PMCID: PMC7599337.
Wolf NI, Breur M, Plug B, Beerepoot S, Westerveld ASR, van Rappard DF, de Vries SI, Kole MHP, Vanderver A, van der Knaap MS, Lindemans CA, van Hasselt PM, Boelens JJ, Matzner U, Gieselmann V, Bugiani M. Metachromatic leukodystrophy and transplantation: remyelination, no cross-correction. Ann Clin Transl Neurol. 2020 Feb;7(2):169-180. doi: 10.1002/acn3.50975. Epub 2020 Jan 22. PMID: 31967741.
Hanemaaijer NA, Popovic MA, Wilders X, Grasman S, Pavón Arocas O, Kole MH. Ca2+ entry through NaV channels generates submillisecond axonal Ca2+signaling. Elife. 2020 Jun 17;9:e54566. doi:10.7554/eLife.54566. PMID: 32553116; PMCID: PMC7380941.
2019
Cohen CCH, Popovic MA, Klooster J, Weil MT, Möbius W, Nave KA, Kole MHP. Saltatory Conduction along Myelinated Axons Involves a Periaxonal Nanocircuit. Cell. 2020 Jan 23;180(2):311-322.e15. doi: 10.1016/j.cell.2019.11.039. Epub 2019 Dec 26. PMID: 31883793.
Byczkowicz N, Eshra A, Montanaro J, Trevisiol A, Hirrlinger J, Kole MH, Shigemoto R, Hallermann S. HCN channel-mediated neuromodulation can control action potential velocity and fidelity in central axons. Elife. 2019 Sep 9;8:e42766. doi: 10.7554/eLife.42766. PMID: 31496517.
Battefeld A, Popovic MA, van der Werf D, Kole MHP. A Versatile and Open- Source Rapid LED Switching System for One-Photon Imaging and Photo-Activation. Front Cell Neurosci. 2019 Jan 17;12:530. doi: 10.3389/fncel.2018.00530. PMID: 30705622.
Battefeld A, Popovic MA, de Vries SI, Kole MHP. High-Frequency Microdomain Ca<sup>2+</sup> Transients and Waves during Early Myelin Internode Remodeling. Cell Rep. 2019 Jan 2;26(1):182-191.e5. doi: 10.1016/j.celrep.2018.12.039. PMID: 30605675.
2018
Klok MD, Bugiani M, de Vries SI, Gerritsen W, Breur M, van der Sluis S, Heine VM, Kole MHP, Baron W, van der Knaap MS. Axonal abnormalities in vanishing white matter. Ann Clin Transl Neurol. 2018 Mar 1;5(4):429-444. doi: 10.1002/acn3.540. PMID: 29687020; PMCID: PMC5899913.
Kole MH, Brette R. The electrical significance of axon location diversity. Curr Opin Neurobiol. 2018 Aug;51:52-59. doi: 10.1016/j.conb.2018.02.016. Epub 2018 Mar 10. PMID: 29533849.
Dubey M, Brouwers E, Hamilton EMC, Stiedl O, Bugiani M, Koch H, Kole MHP, Boschert U, Wykes RC, Mansvelder HD, van der Knaap MS, Min R. Seizures and disturbed brain potassium dynamics in the leukodystrophy megalencephalic leukoencephalopathy with subcortical cysts. Ann Neurol. 2018 Mar;83(3):636-649. doi: 10.1002/ana.25190. Epub 2018 Mar 13. PMID: 29466841.
2017
Hamada MS, Popovic MA, Kole MH. Loss of Saltation and Presynaptic Action Potential Failure in Demyelinated Axons. Front Cell Neurosci. 2017 Feb 27;11:45. doi: 10.3389/fncel.2017.00045. PMID: 28289377.
Klok MD, Bugiani M, de Vries SI, Gerritsen W, Breur M, van der Sluis S, Heine VM, Kole MHP, Baron W, van der Knaap MS. Axonal abnormalities in vanishing white matter. Ann Clin Transl Neurol. 2018 Mar 1;5(4):429-444. doi: 10.1002/acn3.540. PMID: 29687020.
Hamada MS, Popovic MS and Kole MHP (2017) Loss of saltation and presynaptic action potential failure in demyelinated axons. Front. Cell. Neurosci. 10.3389/fncel.2017.00045
2016
Popovic M and Kole MHP. Patch-clamp recording from myelinated central axons. In: Advanced Patch-Clamp Analysis for Neuroscientists. Neuromethods Vol. 113. Alon Korngreen (Ed.). Humana Press (DOI: 10.1007/978-1-4939-3411-9.)
Battefeld A, Klooster J, Kole MHP (2016). Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat. Comm. 7:11298.
Hamada MS, Goethals S, de Vries SI, Brette R and Kole MHP (2016) Covariation of axon initial segment location and dendritic tree normalizes the somatic action potential. Proc. Natl. Acad. Sci. 113:14841–14846.
2015
Mustafa S. Hamada and Maarten H.P. Kole. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J Neurosci 34:7272–7286
2014
Battefeld A, Tran BT, Gavrilis J, Cooper EC, Kole MHP (2014) Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J Neurosci 34:3719–3732.
Stadler K, Bierwirth C, Stoenica L, Battefeld A, Reetz O, Mix E, Schuchmann S, Velmans T, Rosenberger K, Brauer AU, Lehnardt S, Nitsch R, Budt M, Wolff T, Kole MHP, Strauss U (2014) Elevation in type I interferons inhibits HCN1 and slows cortical neuronal oscillations. Cerebral Cortex 24:199–210.
2013
Battefeld A, Kole MHP (2013) A passive cable to excite oligodendrocyte precursor glia. The Journal of Physiology 591:4685–4686.
2012
Hallermann S, de Kock CPJ, Stuart GJ, Kole MHP (2012) State and location dependence of action potential metabolic cost in cortical pyramidal neurons. Nat Neurosci 15:1007–1014.
Kole MHP, Stuart GJ (2012) Signal processing in the axon initial segment. Neuron 73:235–247.
2011-2001
2011-2001
Kole MHP (2011) First Node of Ranvier Facilitates High-Frequency Burst Encoding. Neuron 71:671–682.
Kole MHP (2008) Subthalamic firing without an end, but now with a beginning. The Journal of Physiology 586:5603.
Kole MHP, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ (2008) Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci 11:178–186.
Kole MHP, Stuart GJ (2008) Is action potential threshold lowest in the axon? Nat Neurosci 11:1253–1255.
Kole MHP, Letzkus JJ, Stuart GJ (2007) Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55:633–647.
Kole MHP, Brauer AU, Stuart GJ (2007) Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. The Journal of Physiology 578:507–525.
Davie JT, Kole MHP, Letzkus JJ, Rancz EA, Spruston N, Stuart GJ, Häusser M (2006) Dendritic patch-clamp recording. Nat Protoc 1:1235–1247.
Kole MHP, Hallermann S, Stuart GJ (2006) Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci 26:1677–1687.
Buwalda B, Kole MHP, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM (2005) Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neuroscience & Biobehavioral Reviews 29:83–97.
Fuchs E, Czéh B, Kole MHP, Michaelis T, Lucassen PJ (2004) Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol 14 Suppl 5:S481–S490.
Kole MHP, Costoli T, Koolhaas JM, Fuchs E (2004) Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. NSC 125:337–347.
Kole MHP, Czéh B, Fuchs E (2004) Homeostatic maintenance in excitability of tree shrew hippocampal CA3 pyramidal neurons after chronic stress. Hippocampus 14:742–751.
Strauss U, Kole MHP, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA (2004) An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci 19:3048–3058.
Kole MHP, Swan L, Fuchs E (2002) The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci 16:807–816.
Kole MH, Koolhaas JM, Luiten PG, Fuchs E (2001) High-voltage-activated Ca2+ currents and the excitability of pyramidal neurons in the hippocampal CA3 subfield in rats depend on corticosterone and time of day. Neurosci Lett 307:53–56.

