CV – Research – Lab members – Publications
 Dr. Frederik Verweij
Dr. Frederik Verweij
Cell Biology, Neurobiology and Biophysics
Department of Biology
Faculty of Science, Utrecht University
Kruytgebouw, room N501
Padualaan 8, 3584 CH Utrecht
The Netherlands
Email: f.j.verweij@uu.nl
Curriculum Vitae
Frederik Verweij studied Biomedical Sciences at Utrecht University, and moved to the Cancer Center Amsterdam (Amsterdam UMC) where he obtained his PhD in virology in 2009. His postdoctoral training in cell biology was at Institut Curie and the Institute of Psychiatry and Neurosciences of Paris, funded by an EMBO long-term fellowship and Institut National du Cancer (INCa) fellowships respectively. Since September 2021, he started as group leader in the division of Cell Biology, Neurobiology and Biophysics at Utrecht University where he also joined the Center for Living Technologies (CLT). In 2020, he received a KWF Dutch Cancer Society Young Investigator Grant, and in 2022 an NWO-VIDI grant, and ERC-StG grant.
Research summary
Intercellular communication is essential to multicellular life. Our goal is to understand the mechanisms by which the cells of different organs in our body communicate with each other in order to maintain homeostasis. We focus on small nanometer-sized Extracellular Vesicles (EVs), including endosome derived exosomes, secreted by virtually every cell in our body. EVs are bilayer vesicles that shuttle biological cargo from one cell to another, able to reprogram recipient cells. As such, they are implicated as essential messengers in a wide range of physiological and pathological processes, in particular cancer progression.
Due to their small size and a general lack of suitable model systems, many details of EV biology remain obscure. In our lab, we study various facets of this intriguing and important communication pathway from biogenesis to function. We do so by combining the development of smart molecular tools and transparent zebrafish in vivo model systems with state-of-the-art (live) imaging techniques.
Following this approach, we cover aspects down from single-cell up to organismal level to decode molecular mechanisms in source and receiver cells that underly the biological role of this mode of communication in health and disease.
This focus is reflected by two of the main research lines in the lab:
I) The regulation of exosome secretion | Here, the aim is to better understand the regulatory mechanisms of exosome secretion, and how this supports exosome-mediated communication.
II) The role of EVs in colorectal cancer (CRC) progression in vivo (funded by KWF) | This project entails basic research into the biomolecular mechanisms of two key steps of CRC metastasis.
Lab members
| Technician: | |
| Lenny Droesen | l.p.m.droesen@uu.nl |
| Postdocs: | |
| Jelle van den Bor | j.vandenbor@uu.nl |
| Bárbara Adem Ribeiro | b.f.ademribeiro@uu.nl |
| PhD students: | |
| Anna Elizabeth George | a.e.george@uu.nl |
| Elly Soltani (UMCU) | z.soltani@uu.nl |
| Misko Bobeldijk | m.l.bobeldijk@uu.nl |
| Elisa Costanzo (exchange) | elisa.costanzo01@unipa.it |
| Master students: | |
| Daan Roossien | d.p.roossien@students.uu.nl |
| Anais Bini | a.c.bini@students.uu.nl |
| Mariëlle Floor | m.e.floor2@students.uu.nl |
| Andres Jimenez Pelarda | a.jimenezpelarda@students.uu.nl |
| Emma Bundock | e.m.a.bundock@uu.nl |
| Alumni: | |
| Vincenzo Verdi | (PhD Van Niel Lab, IPNP Paris; PostDoc at NIH Bethesda) |
Publications
2024
Viola M, Bebelman MP, Maas RGC, de Voogt WS, Verweij FJ, Seinen CS, de Jager SCA, Vader P, Pegtel DM, Petrus Gerardus Sluijter J. Hypoxia and TNF-alpha modulate extracellular vesicle release from human induced pluripotent stem cell- derived cardiomyocytes. J Extracell Vesicles. 2024 Nov;13(11):e70000. doi: 10.1002/jev2.70000. PMID: 39508403; PMCID: PMC11541862.
Liu J, Verweij FJ, van Niel G, Galli T, Danglot L, Bun P. ExoJ – a Fiji/ImageJ2 plugin for automated spatiotemporal detection and analysis of exocytosis. J Cell Sci. 2024 Oct 15;137(20):jcs261938. doi: 10.1242/jcs.261938. Epub 2024 Oct 23. PMID: 39219469.
Palmulli R, Couty M, Piontek MC, Ponnaiah M, Dingli F, Verweij FJ, Charrin S, Tantucci M, Sasidharan S, Rubinstein E, Kontush A, Loew D, Lhomme M, Roos WH, Raposo G, van Niel G. CD63 sorts cholesterol into endosomes for storage and distribution via exosomes. Nat Cell Biol. 2024 Jul;26(7):1093-1109. doi: 10.1038/s41556-024-01432-9. Epub 2024 Jun 17. PMID: 38886558.
Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku’ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ; MISEV Consortium; Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024 Feb;13(2):e12404. doi: 10.1002/jev2.12404.
2023
Kapustin A, Tsakali SS, Whitehead M, Chennell G, Wu MY, Molenaar C, Kutikhin A, Bogdanov L, Sinitsky M, Rubina K, Clayton A, Verweij FJ, Pegtel DM, Zingaro S, Lobov A, Zainullina B, Owen D, Parsons M, Cheney RE, Warren D, Humphries MJ, Iskratsch T, Holt M, Shanahan CM. Extracellular vesicles stimulate smooth muscle cell migration by presenting collagen VI. bioRxiv [Preprint]. 2023 Aug 22:2023.08.17.551257. doi: 10.1101/2023.08.17.551257. PMID: 37645762..
Bebelman MP, Setiawan IM, Bergkamp ND, van Senten JR, Crudden C, Bebelman JPM, Verweij FJ, van Niel G, Siderius M, Pegtel DM, Smit MJ. Exosomal release of the virus-encoded chemokine receptor US28 contributes to chemokine scavenging. iScience. 2023 Jul 18;26(8):107412. doi: 10.1016/j.isci.2023.107412. PMID: 37575190..
2022
Verweij, F.J., Bebelman, M.P., George, A.E., Couty, M., Bécot, A., Palmulli, R., Heiligenstein, X., Sirés-Campos, J., Raposo, G., Pegtel, DM., van Niel, G. (2022).
ER membrane contact sites support endosomal small GTPase conversion for exosome secretion. Journal of Cell Biology, doi: 10.1083/jcb.202112032.
2021
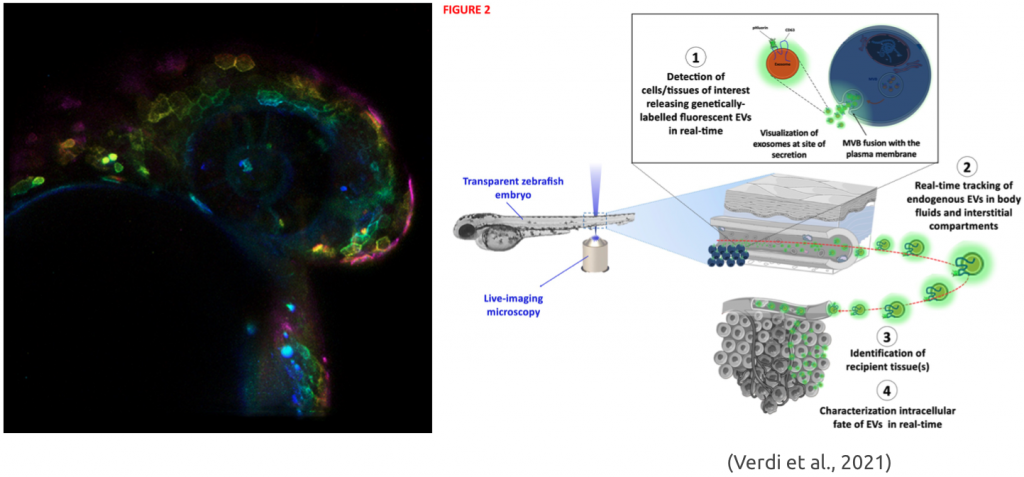
Verdi, V., Bécot, A., Niel, G., & Verweij, F. J. (2021). In vivo imaging of EVs in zebrafish: New perspectives from “the waterside”. FASEB BioAdvances. https://doi.org/10.1096/fba.2021-00081.
Mathieu, M., Névo, N., Jouve, M., Valenzuela, J. I., Maurin, M., Verweij, F. J., Palmulli, R., Lankar, D., Dingli, F., Loew, D., Rubinstein, E., Boncompain, G., Perez, F., & Théry, C. (2021). Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nature Communications, 12(1), [4389]. https://doi.org/10.1038/s41467-021-24384-2.
Androuin, A., Verweij, F. J., & van Niel, G. (2021). Zebrafish as a preclinical model for Extracellular Vesicle-based therapeutic development. Advanced Drug Delivery Reviews, 176, [113815]. https://doi.org/10.1016/j.addr.2021.05.025.
Verweij, F. J., Balaj, L., Boulanger, C. M., Carter, D. R. F., Compeer, E. B., D’Angelo, G., El Andaloussi, S., Goetz, J. G., Gross, J. C., Hyenne, V., Krämer-Albers, E-M., Lai, C. P., Loyer, X., Marki, A., Momma, S., Nolte-‘t Hoen, E. N. M., Pegtel, D. M., Peinado, H., Raposo, G., … van Niel, G. (2021). The power of imaging to understand extracellular vesicle biology in vivo. Nature Methods. https://doi.org/10.1038/s41592-021-01206-3.
2020
Thouvenin, O., Keiser, L., Cantaut-Belarif, Y., Carbo-Tano, M., Verweij, F., Jurisch-Yaksi, N., Bardet, P-L., van Niel, G., Gallaire, F., & Wyart, C. (2020). Origin and role of the cerebrospinal fluid bidirectional flow in the central canal. eLife, 9, [e47699]. https://doi.org/10.7554/eLife.47699.
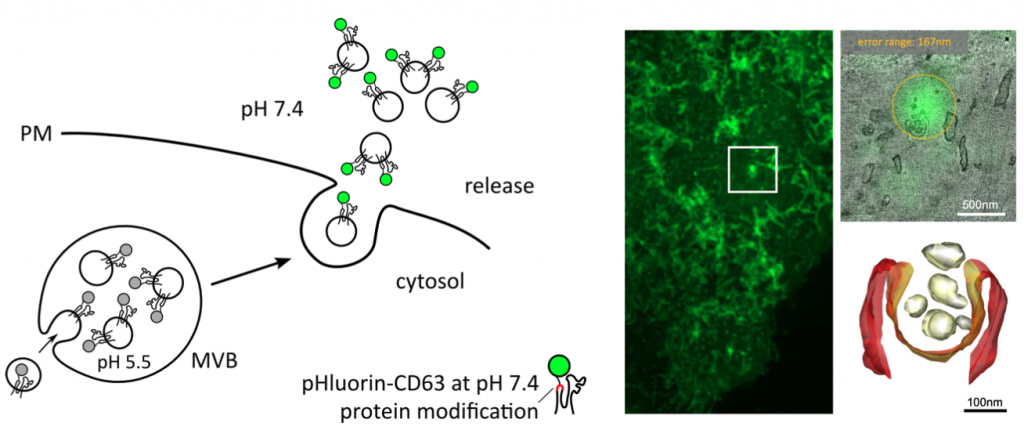
Bebelman, M. P., Bun, P., Huveneers, S., van Niel, G., Pegtel, D. M., & Verweij, F. J. (2020). Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells. Nature protocols, 15(1), 102-121. https://doi.org/10.1038/s41596-019-0245-4.
2019
Hyenne, V., Ghoroghi, S., Collot, M., Bons, J., Follain, G., Harlepp, S., Mary, B., Bauer, J., Mercier, L., Busnelli, I., Lefebvre, O., Fekonja, N., Garcia-Leon, M. J., Machado, P., Delalande, F., López, A. A., Silva, S. G., Verweij, F. J., van Niel, G., … Goetz, J. G. (2019). Studying the Fate of Tumor Extracellular Vesicles at High Spatiotemporal Resolution Using the Zebrafish Embryo. Developmental Cell, 48(4), 554-572.e7. https://doi.org/10.1016/j.devcel.2019.01.014.
Verweij, F. J., Revenu, C., Arras, G., Dingli, F., Loew, D., Pegtel, D. M., Follain, G., Allio, G., Goetz, J. G., Zimmermann, P., Herbomel, P., Del Bene, F., Raposo, G., & van Niel, G. (2019). Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Developmental Cell, 48(4), 573-589.e4. https://doi.org/10.1016/j.devcel.2019.01.004.
Verweij, F. J., Hyenne, V., Van Niel, G., & Goetz, J. G. (2019). Extracellular Vesicles: Catching the Light in Zebrafish. Trends in Cell Biology, 29(10), 770-776. https://doi.org/10.1016/j.tcb.2019.07.007.
2018
Verweij, F. J., Bebelman, M. P., Jimenez, C. R., Garcia-Vallejo, J. J., Janssen, H., Neefjes, J., Knol, J. C., de Goeij-de Haas, R., Piersma, S. R., Baglio, S. R., Verhage, M., Middeldorp, J. M., Zomer, A., van Rheenen, J., Coppolino, M. G., Hurbain, I., Raposo, G., Smit, M. J., Toonen, R. F. G., … Pegtel, D. M. (2018). Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. Journal of Cell Biology, 217(3), 1129-1142. https://doi.org/10.1083/jcb.201703206.
2016
Bijnsdorp, IV., Hodzic, J., Lagerweij, T., Westerman, B., Krijgsman, O., Broeke, J., Verweij, F., Nilsson, RJ., Rozendaal, L., van, B. VW., van, M. JA., & Geldof, AA. (2016). miR-129-3p controls centrosome number in metastatic prostate cancer cells by repressing CP110. Oncotarget. https://doi.org/10.18632/oncotarget.7572.
2015
Baglio, S. R., Rooijers, K., Koppers-Lalic, D., Verweij, F. J., Pérez Lanzón, M., Zini, N., Naaijkens, B., Perut, F., Niessen, H. W. M., Baldini, N., & Pegtel, D. M. (2015). Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem cell research & therapy, 6, 127. https://doi.org/10.1186/s13287-015-0116-z.
Verweij, F. J., de Heus, C., Kroeze, S., Cai, H., Kieff, E., Piersma, S. R., Jimenez, C. R., Middeldorp, J. M., & Pegtel, D. M. (2015). Exosomal sorting of the viral oncoprotein LMP1 is restrained by TRAF2 association at signalling endosomes. Journal of Extracellular Vesicles, 4, 26334. https://doi.org/10.3402/jev.v4.26334.
Zomer, A., Maynard, C., Verweij, F. J., Kamermans, A., Schäfer, R., Beerling, E., Schiffelers, R. M., de Wit, E., Berenguer, J., Ellenbroek, S. I. J., Wurdinger, T., Pegtel, D. M., & van Rheenen, J. (2015). In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell, 161(5), 1046-1057. https://doi.org/10.1016/j.cell.2015.04.042.
2013
Verweij, F. J., van Eijndhoven, M. A. J., Middeldorp, J., & Pegtel, D. M. (2013). Analysis of viral microRNA exchange via exosomes in vitro and in vivo. Methods in Molecular Biology, 1024, 53-68. https://doi.org/10.1007/978-1-62703-453-1_.
2012
Verweij, F. J., Middeldorp, J. M., & Pegtel, D. M. (2012). Intracellular signaling controlled by the endosomal-exosomal pathway. Communicative & Integrative Biology, 5(1), 88-93. https://doi.org/10.4161/cib.18452.
2011
Verweij, F. J., van Eijndhoven, M. A. J., Hopmans, E. S., Vendrig, T., Wurdinger, T., Cahir-McFarland, E., Kieff, E., Geerts, D., van der Kant, R., Neefjes, J., Middeldorp, J. M., & Pegtel, D. M. (2011). LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO Journal, 30(11), 2115-29. https://doi.org/10.1038/emboj.2011.123.





 Anna Akhmanova:
Cellular Dynamics
Anna Akhmanova:
Cellular Dynamics Lukas Kapitein: Biophysics
Lukas Kapitein: Biophysics Florian Berger:
Theoretical Biophysics
Florian Berger:
Theoretical Biophysics Agathe Chaigne:
Cell division dynamics
Agathe Chaigne:
Cell division dynamics Ginny Farías:
Neuronal Organelle Dynamics
Ginny Farías:
Neuronal Organelle Dynamics Martin Harterink:
C. elegans neurobiology
Martin Harterink:
C. elegans neurobiology Casper Hoogenraad: Molecular Neuroscience
Casper Hoogenraad: Molecular Neuroscience Maarten Kole:
Axonal Signalling
Maarten Kole:
Axonal Signalling Sabrina Oliveira:
Molecular Targeted Therapies
Sabrina Oliveira:
Molecular Targeted Therapies Ihor Smal: Image Analysis, Smart Microscopy and AI
Ihor Smal: Image Analysis, Smart Microscopy and AI Frederik Verweij:
Extracellular Vesicle Biology
Frederik Verweij:
Extracellular Vesicle Biology