Mission
The Utrecht Nanobody Facility (UNF) aims to provide support to academic researchers interested in nanobody technology. We provide advice and expertise for development of new nanobodies or new applications with existing nanobodies. In a collaborative set-up we provide the technology for the selection, production, functionalization, and applications of nanobodies. We offer technology for the functionalization of nanobodies using different site-specific conjugation methods of fluorophores (Alexa, Atto, NIR dyes etc.), drugs, nanoparticles etc. Functionalized nanobodies are excellent tracers for imaging purposes and in collaboration with the Biology Imaging Center we provide for single molecule imaging, super-resolution light microscopy, and in vivo molecular imaging.
Technology
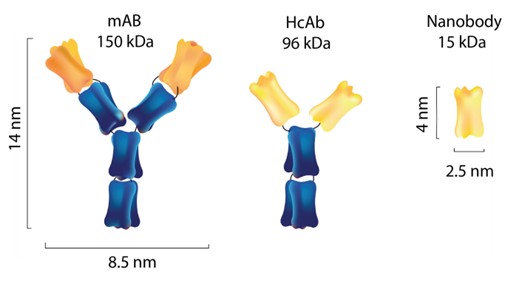
Nanobodies are small antibody fragments (15 kDa) derived from camelid heavy chain antibodies. These single domain antibodies are uniquely adaptable tools. Nanobodies can be selected from (custom built) immune libraries, or alternatively synthetic libraries, using phage display. Extensive equipment is available for the thorough characterization of the nanobodies. Important parameters are production yield, stability, specificity, binding affinity, and selectivity in vivo. Nanobodies can be produced at small scale and equipment is available for the large scale production both from E. coli and HEK cells.
Applications
Nanobodies can be used for different applications, such as stabilization of protein conformation for X-ray crystallography and cryo-electron microscopy, protein or vesicle purification, in vitro imaging (both light- and electron- microscopy), as biosensors, and for in vivo imaging. Furthermore, nanobodies can be employed for therapeutic applications, for instance: as antagonists, conjugated to drugs for cancer therapy or fibrosis, as antivirals, for targeted protein degradation, conjugated to nanoparticles carrying drugs, or in immune therapies such as nanobody-based T cell engagers or chimeric antigen receptor T cells.
Partners
- For the generation of immune libraries we collaborate with our commercial partner, QVQ.
- Since 2021, UNF has been embedded within the Centre for Living Technologies, a cross-institute consortium for synthetic biology within the EWUU Alliance.
- Since 2024, the UNF is sharing facilities with the Biotechnology Student Research HUB, a laboratory dedicated for bachelor projects often connected to Nanobodies
Examples of services
- Production of immune libraries (with QVQ)
- Selection of nanobodies that bind with high specificity and affinity to a target (usually <10 nM)
- Selection of nanobodies from synthetic libraries
- Production and purification at different yield using E. coli or mammalian HEK cells
- Fusion to different tags such as myc, his, EPEA, FLAG, GFP, RFP, among other
- Site-directed conjugation to fluorophores (Alexa, Atto, NIR dyes), biotin or nanoparticles
- Engineering of nanobodies in different formats, including bivalent, bispecific, trimeric, biparatopic
- Typical time lines are three months for immunization and library construction and 1-2 months for selection, though subjected to availability
- Costs may be involved, depending on the set-up of the collaboration
Please contact Sabrina Oliveira for more details.
References
Additional literature and examples of successful application of nanobodies:
1. Nanobody selection technology
Roovers RC, Laeremans T, Huang L, De Taeye S, Verkleij AJ, Revets H, de Haard HJ, van Bergen en Henegouwen PM. Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Nanobodies. Cancer Immunol Immunother. 2007 Mar;56(3):303-317. doi: 10.1007/s00262-006-0180-4.
2. Nanobodies for Cryo-EM
Wout Oosterheert, Katerina T. Xenaki, Viviana Neviani, Wouter Pos, Sofia Doulkeridou, Jip Manshande, Nicholas M. Pearce, Loes M. J. Kroon-Batenburg, Martin Lutz, Paul M. P. van Bergen en Henegouwen, Piet Gros Implications for tetraspanin-enriched microdomain assembly based on structures of CD9 with EWI-F. doi:https://doi.org/10.1101/2020.06.02.130047
3. Nanobodies for high resolution light microscopy
Mikhaylova M, Cloin BM, Finan K, van den Berg R, Teeuw J, Kijanka MM, Sokolowski M, Katrukha EA, Maidorn M, Opazo F, Moutel S, Vantard M, Perez F, van Bergen en Henegouwen PM, Hoogenraad CC, Ewers H, Kapitein LC. Resolving bundled microtubules using anti-tubulin nanobodies. Nat Commun. 2015 Aug 11;6:7933. doi: 10.1038/ncomms8933.
4. Nanobodies for FRET
Hofman, E.G., Bader, A.N., Ruonala, M.O., van den Heuvel, D., Roovers, R.C., Verkleij, A.J., Gerritsen, H.C., and van Bergen en Henegouwen, P.M.P. (2008). EGF induces coalescence of different lipid rafts. J. Cell Science 121: 2519-2528.
5. Nanobodies for whole body imaging
Vosjan MJ, Perk LR, Roovers RC, Visser GW, Stigter-van Walsum M, van Bergen En Henegouwen PM, van Dongen GA. Facile labelling of an anti-epidermal growth factor receptor Nanobody with 68Ga via a novel bifunctional desferal chelate for immuno-PET. Eur J Nucl Med Mol Imaging. 2011 Apr;38(4):753-63. doi: 10.1007/s00259-010-1700-1. Epub 2011 Jan 6. PMID: 21210114
6. Nanobodies for therapy
Xenaki KT, Dorrestijn B, Muns JA, Adamzek K, Doulkeridou S, Houthoff H, Oliveira S, van Bergen En Henegouwen PM. Homogeneous tumor targeting with a single dose of HER2-targeted albumin-binding domain-fused nanobody-drug conjugates results in long-lasting tumor remission in mice. Theranostics. 2021 Mar 13;11(11):5525-5538. doi: 10.7150/thno.57510. eCollection 2021.
7. Nanobodies for biosensors
Su R, Wu YT, Doulkeridou S, Qiu X, Sørensen TJ, Susumu K, Medintz IL, van Bergen En Henegouwen PMP, Hildebrandt N. (2022) A Nanobody-on-Quantum Dot Displacement Assay for Rapid and Sensitive Quantification of the Epidermal Growth Factor Receptor (EGFR). Angew Chem Int Ed Engl. 2022 Aug 15;61(33):e202207797. doi: 10.1002/anie.202207797.
8. Nanobodies for targeted protein degradation
Stam JC, de Maat S, de Jong D, Arens M, van Lint F, Gharu L, van Roosmalen MH, Roovers RC, Strokappe NM, Wagner R, Kliche A, de Haard HJ, van Bergen En Henegouwen PM, Nijhuis M, Verrips CT. Directing HIV-1 for degradation by non-target cells, using bi-specific single-chain llama antibodies. Sci Rep. 2022 Aug 4;12(1):13413. doi: 10.1038/s41598-022-15993-y.
