CV – Research – Lab members – Publications

Prof. Dr. Anna Akhmanova
Cell Biology, Neurobiology and Biophysics
Faculty of Science, Utrecht University
Visiting address:
Kruytgebouw, room O503
Padualaan 8, 3584 CH, Utrecht
The Netherlands
Mailing address:
Kruytgebouw, room O503
Winthontlaan 30C, 3526KV Utrecht
The Netherlands
Tel. 31-(0)30-253 2328
e-mail: a.akhmanova@uu.nl
Curriculum Vitae
Anna Akhmanova studied biochemistry and molecular biology at the Moscow State University. She received her PhD in 1997 at the University of Nijmegen. She worked as a post-doc at the Department of Microbiology and Evolutionary Biology at the University of Nijmegen and at the Department of Cell Biology at the Erasmus Medical Center in Rotterdam. In 2001, she has started her own research group at the Department of Cell Biology at the Erasmus Medical Center. Since 2011, Anna Akhmanova is professor of Cell Biology at Utrecht University.
Currently, Anna Akhmanova is co-chair of the Division of Cell Biology, Neurobiology and Biophysics, Director of the Institute of Biodynamics and Biocomplexity and Chair of the Science for Life community of the Utrecht University Strategic Theme Life Sciences
Anna Akhmanova is a recipient of the ALW Vernieuwingsimpuls VIDI (2001) and VICI awards (2007), an ERC Synergy grant (2013 and 2022), and Spinoza Prize (2018). In 2022 the Ministry of Education, Culture and Science has awarded a Gravitation grant to the project IMAGINE! (Innovative Microscopy and Guidance of cells In their Native Environment), a highly collaborative 10-year project led by Anna Akhmanova.
- Member of the European Molecular Biology Organization (EMBO)
- Member the Royal Netherlands Academy of Arts and Sciences (KNAW)
- Chair of the board of the Netherlands Society for Microscopy (2011-2017)
Current editorial board memberships:
- Journal of Cell Biology, Academic Editor
- Journal of Cell Science, Advisory Board Member
- PLoS Biology, Academic Editor
A pdf of a short CV can be downloaded here.
Research summary
My group studies cytoskeletal organisation and trafficking processes, which contribute to cell polarisation, differentiation, vertebrate development and human disease. We are interested in understanding, at a systems level, how different aspects of cell architecture are coordinated.
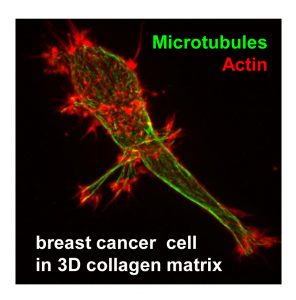 The main focus of our studies is the microtubule cytoskeleton. Our research relies on combining high-resolution live cell imaging and quantitative analysis of cytoskeletal dynamics, in vitro reconstitution of cytoskeleton-based processes and different methods of identification of protein-protein interactions (in vitro binding studies, pull-down assays and mass spectrometry-based protein identification). In addition to conventional 2D cell cultures, we use 3D cell culture models and perform high-speed high-resolution imaging of these cultures. In collaboration with mathematicians we are working on development of automated analysis and modelling of cytoskeletal organisation and transport.
The main focus of our studies is the microtubule cytoskeleton. Our research relies on combining high-resolution live cell imaging and quantitative analysis of cytoskeletal dynamics, in vitro reconstitution of cytoskeleton-based processes and different methods of identification of protein-protein interactions (in vitro binding studies, pull-down assays and mass spectrometry-based protein identification). In addition to conventional 2D cell cultures, we use 3D cell culture models and perform high-speed high-resolution imaging of these cultures. In collaboration with mathematicians we are working on development of automated analysis and modelling of cytoskeletal organisation and transport.
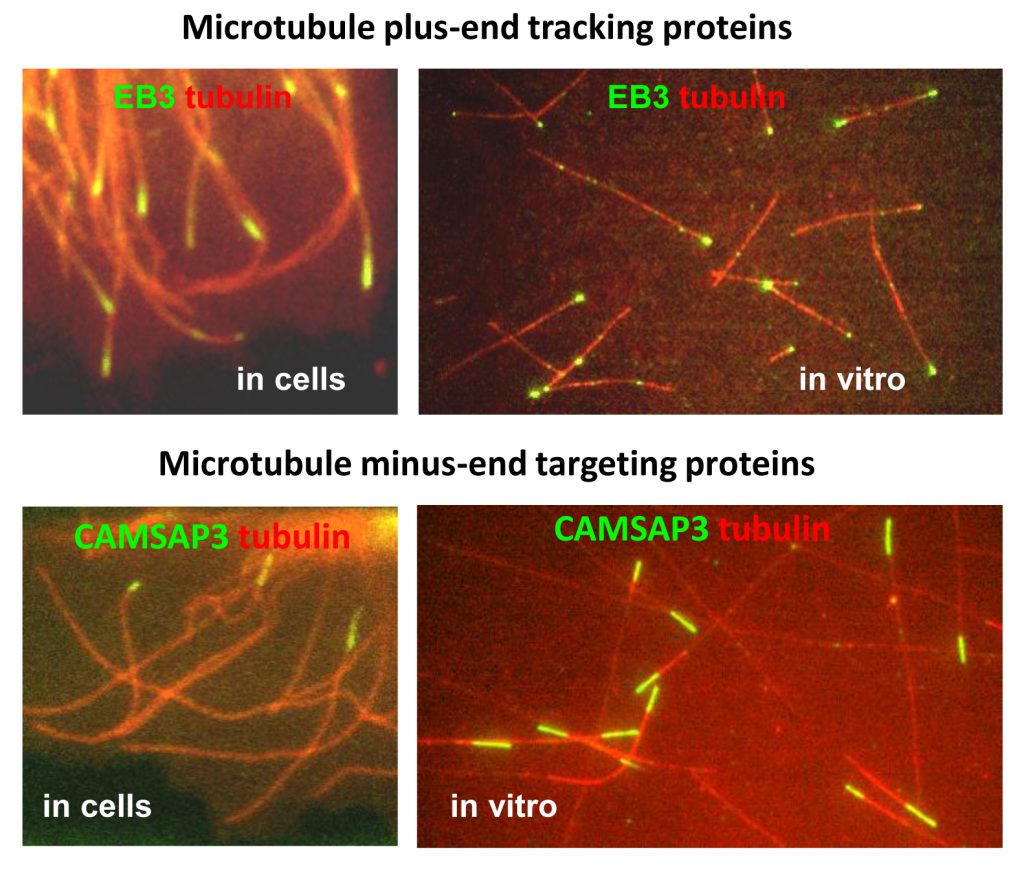
One of the main topics of our research is the structure and function of proteins which bind to the two ends of microtubules, the plus- and the minus-end. We are interested in biochemical activities and functions of microtubule plus-end tracking proteins (+TIPs) – factors that associate specifically with the growing microtubule ends, regulate microtubule dynamics and their interactions with various cellular structures, such as the actin cytoskeleton, focal adhesions and cell-cell contacts. We study how +TIPs help cells to polarise and move or form specialised microtubule-based structures such as cilia and centrioles.
We also investigate the proteins that specifically target and organise microtubule minus ends. Using in vitro reconstitution assays and experiments in cells, we study microtubule nucleation, anchoring and release by the γ-tubulin ring complex. We explore the organization and function of different microtubule-organizing centers and try to understand how minus-end targeting proteins control the geometry of microtubule networks in differentiated cells, such as epithelial and muscle cells
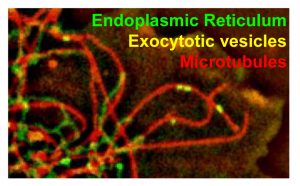
In another line of study, we investigate molecular mechanisms of microtubule-based vesicle transport. We have previously identified several adaptors, such as Bicaudal D, that link microtubule motors, dyneins and kinesins, to their cargo and developed novel assays to demonstrate specific functions of these proteins in recruitment of motor proteins to membranes. Using CRIPSPR/Cas9 gene editing, we are currently dissecting the redundancy between different microtubule motors responsible for vesicle transport in mammalian cell.
We also develop new methods to study microtubules. For example, we have recently designed an optogenetic tool, opto-katanin, that makes it possible to disassemble microtubules in a small cell region while preserving the rest of the microtubule network.
Microtubule-based processes are essential for cell division, normal function of large cells such as neurons and intracellular transport of pathogens; therefore, our studies are relevant for combating abnormal cell proliferation, neurodegeneration and infectious diseases. Microtubule-targeting agents are extensively used to treat cancer and inflammation, and are considered for therapeutic applications in neurodegenerative disorders. We participate in studies aimed at optimising these therapies and use fluorescent derivatives of microtubule-targeting agents to understand their effects on microtubules.
Lab members
| Scientific Staff | |
| Ilya Grigoriev | i.s.grigoriev@uu.nl |
| Technician | |
| Bart de Haan | b.dehaan@uu.nl |
| Postdocs | |
| Joyce Meiring | j.c.m.meiring@uu.nl |
| Yinlong Song | y.song4@uu.nl |
| Benjamin Bouchet | b.bouchet@uu.nl |
| Dipti Rai | d.rai@uu.nl |
| PhD students | |
| Radosław Birger | r.j.birger@uu.nl |
| Emma van Grinsven |
e.j.vangrinsven@uu.nl |
| Lilian Sluimer | l.m.sluimer@uu.nl |
| Saishree Iyer | s.s.iyer@uu.nl |
| Robin Hoogebeen | r.a.hoogebeen@uu.nl |
| Ruijie Liu | r.liu1@uu.nl |
| Elena Radul | e.radul@uu.nl |
| Niels Pagani | n.a.pagani@uu.nl |
| Master students | |
| Jonathan Slejfer | j.c.slejfer@students.uu.nl |
| Quinten Kleijnen |
Publications
For the most up-to-date list of publication click here.
Research Articles – Reviews
Research Articles
2024
de Jager L, Jansen KI, Hoogebeen R, Akhmanova A, Kapitein LC, Förster F, Howes SC. StableMARK-decorated microtubules in cells have expanded lattices. J Cell Biol. 2025 Jan 6;224(1):e202206143. doi: 10.1083/jcb.202206143. Epub 2024 Oct 10. PMID: 39387699.
Schmitt C, Mauker P, Vepřek NA, Gierse C, Meiring JCM, Kuch J, Akhmanova A, Dehmelt L, Thorn-Seshold O. A Photocaged Microtubule-Stabilising Epothilone Allows Spatiotemporal Control of Cytoskeletal Dynamics. Angew Chem Int Ed Engl. 2024 Oct 21;63(43):e202410169. doi: 10.1002/anie.202410169. Epub 2024 Sep 17. PMID: 38961560.
Rai D, Song Y, Hua S, Stecker K, Monster JL, Yin V, Stucchi R, Xu Y, Zhang Y, Chen F, Katrukha EA, Altelaar M, Heck AJR, Wieczorek M, Jiang K, Akhmanova A. CAMSAPs and nucleation-promoting factors control microtubule release from γ-TuRC. Nat Cell Biol. 2024 Mar;26(3):404-420. doi: 10.1038/s41556-024-01366-2.
Epub 2024 Feb 29. PMID: 38424271; PMCID: PMC10940162
Chiang DY, Verkerk AO, Victorio R, Shneyer BI, van der Vaart B, Jouni M, Narendran N, Kc A, Sampognaro JR, Vetrano-Olsen F, Oh JS, Buys E, de Jonge B, Shah DA, Kiviniemi T, Burridge PW, Bezzina CR, Akhmanova A, MacRae CA. The Role of MAPRE2and Microtubules in Maintaining Normal Ventricular Conduction. Circ Res. 2024 Jan 5;134(1):46-59. doi: 10.1161/CIRCRESAHA.123.323231. Epub 2023 Dec 14. PMID: 38095085.
2023
Damstra HGJ, Passmore JB, Serweta AK, Koutlas I, Burute M, Meye FJ, Akhmanova A, Kapitein LC. GelMap: intrinsic calibration and deformation mapping for expansion microscopy. Nat Methods. 2023 Oct;20(10):1573-1580. doi: 10.1038/s41592-023-02001-y. Epub 2023 Sep 18. PMID: 37723243
Li Y, Kučera O, Cuvelier D, Rutkowski DM, Deygas M, Rai D, Pavlovič T, Vicente FN, Piel M, Giannone G, Vavylonis D, Akhmanova A, Blanchoin L, Théry M. Compressive forces stabilize microtubules in living cells. Nat Mater. 2023 Jul;22(7):913-924. doi: 10.1038/s41563-023-01578-1. Epub 2023 Jun 29. PMID: 37386067.
Damstra HGJ, Mohar B, Eddison M, Akhmanova A, Kapitein LC, Tillberg PW. Ten-fold Robust Expansion Microscopy. Bio Protoc. 2023 Jun 20;13(12):e4698. doi: 10.21769/BioProtoc.4698. PMID: 37397797; PMCID: PMC10308184.
Vennin C, Cattaneo CM, Bosch L, Vegna S, Ma X, Damstra HGJ, Martinovic M, Tsouri E, Ilic M, Azarang L, van Weering JRT, Pulver E, Zeeman AL, Schelfhorst T, Lohuis JO, Rios AC, Dekkers JF, Akkari L, Menezes R, Medema R, Baglio SR, Akhmanova A, Linn SC, Lemeer S, Pegtel DM, Voest EE, van Rheenen J. Taxanes trigger cancer cell killing in vivo by inducing non-canonical T cell cytotoxicity. Cancer Cell. 2023 Jun 12;41(6):1170-1185.e12. doi: 10.1016/j.ccell.2023.05.009. PMID: 37311414.
van den Berg CM, Volkov VA, Schnorrenberg S, Huang Z, Stecker KE, Grigoriev I, Gilani S, Frikstad KM, Patzke S, Zimmermann T, Dogterom M, Akhmanova A. CSPP1 stabilizes growing microtubule ends and damaged lattices from the luminal side. J Cell Biol. 2023 Apr 3;222(4):e202208062. doi: 10.1083/jcb.202208062. Epub 2023 Feb 8. PMID: 36752787; PMCID: PMC9948759.
Nick Maleki A, Huis In ‘t Veld PJ, Akhmanova A, Dogterom M, Volkov VA. Estimation of microtubule-generated forces using a DNA origami nanospring. J Cell Sci. 2023 Mar 1;136(5):jcs260154. doi: 10.1242/jcs.260154. Epub 2022 Oct 5. PMID: 36074043.
2022
Damstra HGJ, Mohar B, Eddison M, Akhmanova A, Kapitein LC, Tillberg PW. Correction: Visualizing cellular and tissue ultrastructure using Ten-fold Robust Expansion Microscopy (TREx). Elife. 2022 Nov 29;11:e85169. doi: 10.7554/eLife.85169. PMID: 36444779; PMCID: PMC9708063.
Rushworth JL, Thawani AR, Fajardo-Ruiz E, Meiring JCM, Heise C, White AJP, Akhmanova A, Brandt JR, Thorn-Seshold O, Fuchter MJ. [5]-Helistatins: Tubulin-Binding Helicenes with Antimitotic Activity. JACS Au. 2022 Oct 19;2(11):2561-2570. doi: 10.1021/jacsau.2c00435. PMID: 36465552; PMCID: PMC9709948.
Meiring JCM, Grigoriev I, Nijenhuis W, Kapitein LC, Akhmanova A. Opto-katanin, an optogenetic tool for localized, microtubule disassembly. Curr Biol. 2022 Nov 7;32(21):4660-4674.e6. doi: 10.1016/j.cub.2022.09.010. Epub 2022 Sep 28. PMID: 36174574.
Dusza HM, Katrukha EA, Nijmeijer SM, Akhmanova A, Vethaak AD, Walker DI, Legler J. Uptake, Transport, and Toxicity of Pristine and Weathered Micro- and Nanoplastics in Human Placenta Cells. Environ Health Perspect. 2022 Sep;130(9):97006. doi: 10.1289/EHP10873. Epub 2022 Sep 21. PMID: 36129437; PMCID: PMC9491364.
Willekers S, Tessadori F, van der Vaart B, Henning HH, Stucchi R, Altelaar M, Roelen BAJ, Akhmanova A, Bakkers J. The centriolar satellite protein Cfap53 facilitates formation of the zygotic microtubule organizing center in the zebrafish embryo. Development. 2022 Aug 15;149(16):dev198762. doi: 10.1242/dev.198762. Epub 2022 Aug 18. PMID: 35980365; PMCID: PMC9481976.
Chen F, Wu J, Iwanski MK, Jurriens D, Sandron A, Pasolli M, Puma G, Kromhout JZ, Yang C, Nijenhuis W, Kapitein LC, Berger F, Akhmanova A. Self-assembly of pericentriolar material in interphase cells lacking centrioles. Elife. 2022 Jul 5;11:e77892. doi: 10.7554/eLife.77892. PMID: 35787744; PMCID: PMC9307276.
Morthorst SK, Nielsen C, Farinelli P, Anvarian Z, Rasmussen CBR, Serra-Marques A, Grigoriev I, Altelaar M, Fürstenberg N, Ludwig A, Akhmanova A, Christensen ST, Pedersen LB. Angiomotin isoform 2 promotes binding of PALS1 to KIF13B at primary cilia and regulates ciliary length and signaling. J Cell Sci. 2022 Jun 15;135(12):jcs259471. doi: 10.1242/jcs.259471. Epub 2022 Jun 24. PMID: 35673984.
Gao L, Meiring JCM, Varady A, Ruider IE, Heise C, Wranik M, Velasco CD, Taylor JA, Terni B, Weinert T, Standfuss J, Cabernard CC, Llobet A, Steinmetz MO, Bausch AR, Distel M, Thorn-Seshold J, Akhmanova A, Thorn-Seshold O. In Vivo Photocontrol of Microtubule Dynamics and Integrity, Migration and Mitosis, by the Potent GFP-Imaging-Compatible Photoswitchable Reagents SBTubA4P and SBTub2M. J Am Chem Soc. 2022 Mar 30;144(12):5614-5628. doi: 10.1021/jacs.2c01020. Epub 2022 Mar 15. PMID: 35290733; PMCID: PMC8972266.
Alkemade C, Wierenga H, Volkov VA, Preciado López M, Akhmanova A, Ten Wolde PR, Dogterom M, Koenderink GH. Cross-linkers at growing microtubule ends generate forces that drive actin transport. Proc Natl Acad Sci U S A. 2022 Mar 15;119(11):e2112799119. doi: 10.1073/pnas.2112799119. Epub 2022 Mar 10. PMID: 35271394; PMCID: PMC8931237.
Gao L, Meiring JCM, Heise C, Rai A, Müller-Deku A, Akhmanova A, Thorn-Seshold J, Thorn-Seshold O. Photoswitchable Epothilone-Based Microtubule Stabilisers Allow GFP-Imaging-Compatible, Optical Control over the Microtubule Cytoskeleton. Angew Chem Int Ed Engl. 2022 Mar 1;61(10):e202114614. doi: 10.1002/anie.202114614. Epub 2022 Jan 20. PMID: 34902214; PMCID: PMC9305116.
Noordstra I, van den Berg CM, Boot FWJ, Katrukha EA, Yu KL, Tas RP, Portegies S, Viergever BJ, de Graaff E, Hoogenraad CC, de Koning EJP, Carlotti F, Kapitein LC, Akhmanova A. Organization and dynamics of the cortical complexes controlling insulin secretion in β-cells. J Cell Sci. 2022 Feb 1;135(3):jcs259430. doi: 10.1242/jcs.259430. Epub 2022 Feb 3. PMID: 35006275; PMCID: PMC8918791.
2021
Rai A, Liu T, Katrukha EA, Estévez-Gallego J, Manka SW, Paterson I, Díaz JF, Kapitein LC, Moores CA, Akhmanova A. Lattice defects induced by microtubule-stabilizing agents exert a long-range effect on microtubule growth by promoting catastrophes. Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2112261118. doi: 10.1073/pnas.2112261118. PMID: 34916292; PMCID: PMC8713758.
Sailer A, Meiring JCM, Heise C, Pettersson LN, Akhmanova A, Thorn-Seshold J, Thorn-Seshold O. Pyrrole Hemithioindigo Antimitotics with Near-Quantitative Bidirectional Photoswitching that Photocontrol Cellular Microtubule Dynamics with Single-Cell Precision*. Angew Chem Int Ed Engl. 2021 Oct 25;60(44):23695-23704. doi: 10.1002/anie.202104794. Epub 2021 Oct 1. PMID: 34460143; PMCID: PMC8596636.
Cowell AR, Jacquemet G, Singh AK, Varela L, Nylund AS, Ammon YC, Brown DG, Akhmanova A, Ivaska J, Goult BT. Talin rod domain-containing protein 1 (TLNRD1) is a novel actin-bundling protein which promotes filopodia formation. J Cell Biol. 2021 Sep 6;220(9):e202005214. doi: 10.1083/jcb.202005214. Epub 2021 Jul 15. PMID: 34264272; PMCID: PMC8287531.
Buijs RR, Hummel JJA, Burute M, Pan X, Cao Y, Stucchi R, Altelaar M, Akhmanova A, Kapitein LC, Hoogenraad CC. WDR47 protects neuronal microtubule minus ends from katanin-mediated severing. Cell Rep. 2021 Jul 13;36(2):109371. doi: 10.1016/j.celrep.2021.109371. PMID: 34260930.
Remmelzwaal S, Geisler F, Stucchi R, van der Horst S, Pasolli M, Kroll JR, Jarosinska OD, Akhmanova A, Richardson CA, Altelaar M, Leube RE, Ramalho JJ, Boxem M. BBLN-1 is essential for intermediate filament organization and apical membrane morphology. Curr Biol. 2021 Jun 7;31(11):2334-2346.e9. doi: 10.1016/j.cub.2021.03.069. Epub 2021 Apr 14. PMID: 33857431.
Gros OJ, Damstra HGJ, Kapitein LC, Akhmanova A, Berger F. Dynein self-organizes while translocating the centrosome in T-cells. Mol Biol Cell. 2021 Apr 19;32(9):855-868. doi: 10.1091/mbc.E20-10-0668. Epub 2021 Mar 10. PMID: 33689395; PMCID: PMC8108531.
Gao L, Meiring JCM, Kraus Y, Wranik M, Weinert T, Pritzl SD, Bingham R, Ntouliou E, Jansen KI, Olieric N, Standfuss J, Kapitein LC, Lohmüller T, Ahlfeld J, Akhmanova A, Steinmetz MO, Thorn-Seshold O. A Robust, GFP-Orthogonal Photoswitchable Inhibitor Scaffold Extends Optical Control over the Microtubule Cytoskeleton. Cell Chem Biol. 2021 Feb 18;28(2):228-241.e6. doi: 10.1016/j.chembiol.2020.11.007. Epub 2020 Dec 3. PMID: 33275880.
Luo Y, Xiang S, Paioni AL, Adler A, Hooikaas PJ, Jijumon AS, Janke C, Akhmanova A, Baldus M. Solid-State NMR Spectroscopy for Studying Microtubules and Microtubule-Associated Proteins. Methods Mol Biol. 2021;2305:193-201. doi: 10.1007/978-1-0716-1406-8_10. PMID: 33950391.
2020-2011
2020
Serra-Marques A, Martin M, Katrukha EA, Grigoriev I, Peeters CA, Liu Q, Hooikaas PJ, Yao Y, Solianova V, Smal I, Pedersen LB, Meijering E, Kapitein LC, and Akhmanova A. Concerted action of kinesins KIF5B and KIF13B promotes efficient secretory vesicle transport to microtubule plus ends. Elife 2020, 9: e61302.
Aher A, Rai D, Schaedel L, Gaillard J, John K, Liu Q, Altelaar M, Blanchoin L, Thery M, and Akhmanova A. CLASP Mediates Microtubule Repair by Restricting Lattice Damage and Regulating Tubulin Incorporation. Curr Biol 2020, 30(11): 2175-2183.
Rodriguez-Garcia R, Volkov VA, Chen CY, Katrukha EA, Olieric N, Aher A, Grigoriev I, Lopez MP, Steinmetz MO, Kapitein LC, Koenderink G, Dogterom M, and Akhmanova A. Mechanisms of Motor-Independent Membrane Remodeling Driven by Dynamic Microtubules. Curr Biol 2020, 30(6): 972-987.
Adriaans IE, Hooikaas PJ, Aher A, Vromans MJM, van Es RM, Grigoriev I, Akhmanova A, and Lens SMA. MKLP2 Is a Motile Kinesin that Transports the Chromosomal Passenger Complex during Anaphase. Curr Biol 2020, 30(13): 2628-2637.
Rai A, Liu T, Glauser S, Katrukha EA, Estevez-Gallego J, Rodriguez-Garcia R, Fang WS, Diaz JF, Steinmetz MO, Altmann KH, Kapitein LC, Moores CA, and Akhmanova A. Taxanes convert regions of perturbed microtubule growth into rescue sites. Nat Mater 2020, 19(3): 355-365.
Luo Y, Xiang S, Hooikaas PJ, van Bezouwen L, Jijumon AS, Janke C, Forster F, Akhmanova A, and Baldus M. Direct observation of dynamic protein interactions involving human microtubules using solid-state NMR spectroscopy. Nat Commun 2020, 11(1): 18.
Saraon P, Snider J, Kalaidzidis Y, Wybenga-Groot LE, Weiss K, Rai A, Radulovich N, Drecun L, Vuckovic N, Vucetic A, Wong V, Theriault B, Pham NA, Park JH, Datti A, Wang J, Pathmanathan S, Aboualizadeh F, Lyakisheva A, Yao Z, Wang Y, Joseph B, Aman A, Moran MF, Prakesch M, Poda G, Marcellus R, Uehling D, Samarzija M, Jakopovic M, Tsao MS, Shepherd FA, Sacher A, Leighl N, Akhmanova A, Al-Awar R, Zerial M, and Stagljar I. A drug discovery platform to identify compounds that inhibit EGFR triple mutants. Nat Chem Biol 2020, 16(5): 577-586.
Jost M, Chen Y, Gilbert LA, Horlbeck MA, Krenning L, Menchon G, Rai A, Cho MY, Stern JJ, Prota AE, Kampmann M, Akhmanova A, Steinmetz MO, Tanenbaum ME, and Weissman JS. Pharmaceutical-Grade Rigosertib Is a Microtubule-Destabilizing Agent. Mol Cell 2020, 79(1): 191-198.
Peronne L, Denarier E, Rai A, Prudent R, Vernet A, Suzanne P, Ramirez-Rios S, Michallet S, Guidetti M, Vollaire J, Lucena-Agell D, Ribba AS, Josserand V, Coll JL, Dallemagne P, Diaz JF, Oliva MA, Sadoul K, Akhmanova A, Andrieux A, and Lafanechere L. Two Antagonistic Microtubule Targeting Drugs Act Synergistically to Kill Cancer Cells. Cancers (Basel) 2020, 12(8): :2196.
Yao Y, Smal I, Grigoriev I, Akhmanova A, and Meijering E. Deep-learning method for data association in particle tracking. Bioinformatics 2020, 36(19): 4935-4941.
Muller-Deku A, Meiring JCM, Loy K, Kraus Y, Heise C, Bingham R, Jansen KI, Qu X, Bartolini F, Kapitein LC, Akhmanova A, Ahlfeld J, Trauner D, and Thorn-Seshold O. Photoswitchable paclitaxel-based microtubule stabilisers allow optical control over the microtubule cytoskeleton. Nat Commun 2020, 11(1): 4640.
Gao L, Meiring JCM, Kraus Y, Wranik M, Weinert T, Pritzl SD, Bingham R, Ntouliou E, Jansen KI, Olieric N, Standfuss J, Kapitein LC, Lohmuller T, Ahlfeld J, Akhmanova A, Steinmetz MO, and Thorn-Seshold O. A Robust, GFP-Orthogonal Photoswitchable Inhibitor Scaffold Extends Optical Control over the Microtubule Cytoskeleton. Cell Chem Biol 2020, S2451-9456(20)30470-0.
2019
Hooikaas PJ, Martin M, Muhlethaler T, Kuijntjes GJ, Peeters CAE, Katrukha EA, Ferrari L, Stucchi R, Verhagen DGF, van Riel WE, Grigoriev I, Altelaar AFM, Hoogenraad CC, Rudiger SGD, Steinmetz MO, Kapitein LC, and Akhmanova A. MAP7 family proteins regulate kinesin-1 recruitment and activation. J Cell Biol 2019, 218(4): 1298-1318.
van de Willige D, Hummel JJ, Alkemade C, Kahn OI, Au FK, Qi RZ, Dogterom M, Koenderink GH, Hoogenraad CC, and Akhmanova A. Cytolinker Gas2L1 regulates axon morphology through microtubule-modulated actin stabilization. EMBO Rep 2019, 20(11): e47732.
Freal A, Rai D, Tas RP, Pan X, Katrukha EA, van de Willige D, Stucchi R, Aher A, Yang C, Altelaar AFM, Vocking K, Post JA, Harterink M, Kapitein LC, Akhmanova A, and Hoogenraad CC. Feedback-Driven Assembly of the Axon Initial Segment. Neuron 2019, 104(2): 305-321.
Yu M, Le S, Ammon YC, Goult BT, Akhmanova A, and Yan J. Force-Dependent Regulation of Talin-KANK1 Complex at Focal Adhesions. Nano Lett 2019, 19(9): 5982-5990.
Faltova L, Jiang K, Frey D, Wu Y, Capitani G, Prota AE, Akhmanova A, Steinmetz MO, and Kammerer RA. Crystal Structure of a Heterotetrameric Katanin p60:p80 Complex. Structure 2019, 27(9): 1375-1383.
Pan X, Cao Y, Stucchi R, Hooikaas PJ, Portegies S, Will L, Martin M, Akhmanova A, Harterink M, and Hoogenraad CC. MAP7D2 Localizes to the Proximal Axon and Locally Promotes Kinesin-1-Mediated Cargo Transport into the Axon. Cell Rep 2019, 26(8): 1988-1999.
Jespersen N, Estelle A, Waugh N, Davey NE, Blikstad C, Ammon YC, Akhmanova A, Ivarsson Y, Hendrix DA, and Barbar E. Systematic identification of recognition motifs for the hub protein LC8. Life Sci Alliance 2019, 2(4).
Frikstad KM, Molinari E, Thoresen M, Ramsbottom SA, Hughes F, Letteboer SJF, Gilani S, Schink KO, Stokke T, Geimer S, Pedersen LB, Giles RH, Akhmanova A, Roepman R, Sayer JA, and Patzke S. A CEP104-CSPP1 Complex Is Required for Formation of Primary Cilia Competent in Hedgehog Signaling. Cell Rep 2019, 28(7): 1907-1922.
Atherton J, Luo Y, Xiang S, Yang C, Rai A, Jiang K, Stangier M, Vemu A, Cook AD, Wang S, Roll-Mecak A, Steinmetz MO, Akhmanova A, Baldus M, and Moores CA. Structural determinants of microtubule minus end preference in CAMSAP CKK domains. Nat Commun 2019, 10(1): 5236.
2018
Aher A, Kok M, Sharma A, Rai A, Olieric N, Rodriguez-Garcia R, Katrukha EA, Weinert T, Olieric V, Kapitein LC, Steinmetz MO, Dogterom M, and Akhmanova A. CLASP Suppresses Microtubule Catastrophes through a Single TOG Domain. Dev Cell 2018, 46(1): 40-58.
Martin M, Veloso A, Wu J, Katrukha EA, and Akhmanova A. Control of endothelial cell polarity and sprouting angiogenesis by non-centrosomal microtubules. Elife 2018, 7: e33864.
Fielmich LE, Schmidt R, Dickinson DJ, Goldstein B, Akhmanova A, and van den Heuvel S. Optogenetic dissection of mitotic spindle positioning in vivo. Elife 2018, 7: e38198.
Jiang K, Faltova L, Hua S, Capitani G, Prota AE, Landgraf C, Volkmer R, Kammerer RA, Steinmetz MO, and Akhmanova A. Structural Basis of Formation of the Microtubule Minus-End-Regulating CAMSAP-Katanin Complex. Structure 2018, 26(3): 375-382.
Tas RP, Chen CY, Katrukha EA, Vleugel M, Kok M, Dogterom M, Akhmanova A, and Kapitein LC. Guided by Light: Optical Control of Microtubule Gliding Assays. Nano Lett 2018, 18(12): 7524-7528.
Galmarini CM, Martin M, Bouchet BP, Guillen-Navarro MJ, Martinez-Diez M, Martinez-Leal JF, Akhmanova A, and Aviles P. Plocabulin, a novel tubulin-binding agent, inhibits angiogenesis by modulation of microtubule dynamics in endothelial cells. BMC Cancer 2018, 18(1): 164.
2017
Rezabkova L, Jiang K, Capitani G, Prota AE, Akhmanova A, Steinmetz MO, and Kammerer RA. Structural basis of katanin p60:p80 complex formation. Sci Rep, 2017; 7 (1): 14893.
Liu Q, Remmelzwaal S, Heck AJR, Akhmanova A, and Liu F. Facilitating identification of minimal protein binding domains by cross-linking mass spectrometry. Sci Rep, 2017; 7 (1): 13453.
Atherton J, Jiang K, Stangier MM, Luo Y, Hua S, Houben K, van Hooff JJE, Joseph AP, Scarabelli G, Grant BJ, Roberts AJ, Topf M, Steinmetz MO, Baldus M, Moores CA, and Akhmanova A. A structural model for microtubule minus-end recognition and protection by CAMSAP proteins. Nat Struct Mol Biol, 2017; 24 (11): 931-943.
Jost M, Chen Y, Gilbert LA, Horlbeck MA, Krenning L, Menchon G, Rai A, Cho MY, Stern JJ, Prota AE, Kampmann M, Akhmanova A, Steinmetz MO, Tanenbaum ME, and Weissman JS. Combined CRISPRi/a-Based Chemical Genetic Screens Reveal that Rigosertib Is a Microtubule-Destabilizing Agent. Mol Cell, 2017; 68 (1): 210-223 e6.
Yang C, Wu J, de Heus C, Grigoriev I, Liv N, Yao Y, Smal I, Meijering E, Klumperman J, Qi RZ, and Akhmanova A. EB1 and EB3 regulate microtubule minus end organization and Golgi morphology. J Cell Biol, 2017; 216 (10): 3179-3198.
Schmidt R, Fielmich LE, Grigoriev I, Katrukha EA, Akhmanova A, and van den Heuvel S. Two populations of cytoplasmic dynein contribute to spindle positioning in C. elegans embryos. J Cell Biol, 2017; 216 (9): 2777-2793.
Kumar A, Manatschal C, Rai A, Grigoriev I, Degen MS, Jaussi R, Kretzschmar I, Prota AE, Volkmer R, Kammerer RA, Akhmanova A, and Steinmetz MO. Short Linear Sequence Motif LxxPTPh Targets Diverse Proteins to Growing Microtubule Ends. Structure, 2017; 25 (6): 924-932 e4.
Jiang K, Rezabkova L, Hua S, Liu Q, Capitani G, Altelaar AFM, Heck AJR, Kammerer RA, Steinmetz MO, and Akhmanova A. Microtubule minus-end regulation at spindle poles by an ASPM-katanin complex. Nat Cell Biol, 2017; 19 (5): 480-492.
Gumy LF, Katrukha EA, Grigoriev I, Jaarsma D, Kapitein LC, Akhmanova A, and Hoogenraad CC. MAP2 Defines a Pre-axonal Filtering Zone to Regulate KIF1- versus KIF5-Dependent Cargo Transport in Sensory Neurons. Neuron, 2017; 94 (2): 347-362 e7.
Yao Y, Smal I, Grigoriev I, Martin M, Akhmanova A, and Meijering E. Automated Analysis of Intracellular Dynamic Processes. Methods Mol Biol, 2017; 1563: 209-228.
Katrukha EA, Mikhaylova M, van Brakel HX, van Bergen En Henegouwen PM, Akhmanova A, Hoogenraad CC, and Kapitein LC. Probing cytoskeletal modulation of passive and active intracellular dynamics using nanobody-functionalized quantum dots. Nat Commun, 2017; 8: 14772.
van Riel WE, Rai A, Bianchi S, Katrukha EA, Liu Q, Heck AJ, Hoogenraad CC, Steinmetz MO, Kapitein LC, and Akhmanova A. Kinesin-4 KIF21B is a potent microtubule pausing factor. Elife, 2017; 6.
Bohnacker T, Prota AE, Beaufils F, Burke JE, Melone A, Inglis AJ, Rageot D, Sele AM, Cmiljanovic V, Cmiljanovic N, Bargsten K, Aher A, Akhmanova A, Diaz JF, Fabbro D, Zvelebil M, Williams RL, Steinmetz MO, and Wymann MP. Deconvolution of Buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat Commun, 2017; 8: 14683.
Schou KB, Mogensen JB, Morthorst SK, Nielsen BS, Aleliunaite A, Serra-Marques A, Furstenberg N, Saunier S, Bizet AA, Veland IR, Akhmanova A, Christensen ST, and Pedersen LB. KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling. Nat Commun, 2017; 8: 14177.
Au FK, Jia Y, Jiang K, Grigoriev I, Hau BK, Shen Y, Du S, Akhmanova A, and Qi RZ. GAS2L1 Is a Centriole-Associated Protein Required for Centrosome Dynamics and Disjunction. Dev Cell, 2017; 40 (1): 81-94.
2016
Bianchi S, van Riel WE, Kraatz SH, Olieric N, Frey D, Katrukha EA, Jaussi R, Missimer J, Grigoriev I, Olieric V, Benoit RM, Steinmetz MO, Akhmanova A, and Kammerer RA. Structural basis for misregulation of kinesin KIF21A autoinhibition by CFEOM1 disease mutations. Sci Rep, 2016. 6: 30668.
Bouchet BP, Gough RE, Ammon YC, van de Willige D, Post H, Jacquemet G, Altelaar AM, Heck AJ, Goult BT, and Akhmanova A. Talin-KANK1 interaction controls the recruitment of cortical microtubule stabilizing complexes to focal adhesions. Elife, 2016. 5.
Bouchet BP, Noordstra I, van Amersfoort M, Katrukha EA, Ammon YC, Ter Hoeve ND, Hodgson L, Dogterom M, Derksen PW, and Akhmanova A. Mesenchymal Cell Invasion Requires Cooperative Regulation of Persistent Microtubule Growth by SLAIN2 and CLASP1. Dev Cell, 2016. 39(6): 708-723.
Doodhi H, Prota AE, Rodriguez-Garcia R, Xiao H, Custar DW, Bargsten K, Katrukha EA, Hilbert M, Hua S, Jiang K, Grigoriev I, Yang CP, Cox D, Horwitz SB, Kapitein LC, Akhmanova A, and Steinmetz MO. Termination of Protofilament Elongation by Eribulin Induces Lattice Defects that Promote Microtubule Catastrophes. Curr Biol, 2016. 26(13): 1713-21.
Guesdon A, Bazile F, Buey RM, Mohan R, Monier S, Garcia RR, Angevin M, Heichette C, Wieneke R, Tampe R, Duchesne L, Akhmanova A, Steinmetz MO, and Chretien D. EB1 interacts with outwardly curved and straight regions of the microtubule lattice. Nat Cell Biol, 2016. 18(10): 1102-8.
Kuijpers M, van de Willige D, Freal A, Chazeau A, Franker MA, Hofenk J, Rodrigues RJ, Kapitein LC, Akhmanova A, Jaarsma D, and Hoogenraad CC. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron, 2016. 89(3): 461-71.
Liu Q, Liu F, Yu KL, Tas R, Grigoriev I, Remmelzwaal S, Serra-Marques A, Kapitein LC, Heck AJ, and Akhmanova A. MICAL3 Flavoprotein Monooxygenase Forms a Complex with Centralspindlin and Regulates Cytokinesis. J Biol Chem, 2016. 291(39): 20617-29.
Noordstra I, Liu Q, Nijenhuis W, Hua S, Jiang K, Baars M, Remmelzwaal S, Martin M, Kapitein LC, and Akhmanova A. Control of apico-basal epithelial polarity by the microtubule minus-end-binding protein CAMSAP3 and spectraplakin ACF7. J Cell Sci, 2016. 129(22): 4278-4288.
Portegijs V, Fielmich LE, Galli M, Schmidt R, Munoz J, van Mourik T, Akhmanova A, Heck AJ, Boxem M, and van den Heuvel S. Multisite Phosphorylation of NuMA-Related LIN-5 Controls Mitotic Spindle Positioning in C. elegans. PLoS Genet, 2016. 12(10): e1006291.
Rezabkova L, Kraatz SH, Akhmanova A, Steinmetz MO, and Kammerer RA. Biophysical and Structural Characterization of the Centriolar Protein Cep104 Interaction Network. J Biol Chem, 2016. 291(35): 18496-504.
Sharma A, Aher A, Dynes NJ, Frey D, Katrukha EA, Jaussi R, Grigoriev I, Croisier M, Kammerer RA, Akhmanova A, Gonczy P, and Steinmetz MO. Centriolar CPAP/SAS-4 Imparts Slow Processive Microtubule Growth. Dev Cell, 2016. 37(4): 362-76.
Wu J, de Heus C, Liu Q, Bouchet BP, Noordstra I, Jiang K, Hua S, Martin M, Yang C, Grigoriev I, Katrukha EA, Altelaar AF, Hoogenraad CC, Qi RZ, Klumperman J, and Akhmanova A. Molecular Pathway of Microtubule Organization at the Golgi Apparatus. Dev Cell, 2016. 39(1): 44-60.
2015
van Beuningen SF, Will L, Harterink M, Chazeau A, van Battum EY, Frias CP, Franker MA, Katrukha EA, Stucchi R, Vocking K, Antunes AT, Slenders L, Doulkeridou S, Sillevis Smitt P, Altelaar AF, Post JA, Akhmanova A, Pasterkamp RJ, Kapitein LC, de Graaff E, Hoogenraad CC. TRIM46 Controls Neuronal Polarity and Axon Specification by Driving the Formation of Parallel Microtubule Arrays. Neuron. 2015 Dec 16;88(6):1208-26. doi: 10.1016/j.neuron.2015.11.012. Epub 2015 Dec 6. PubMed PMID: 26671463.
Long Y, Goedhart J, Schneijderberg M, Terpestra I, Shimotohno A, Bouchet BP, Akhmanova A, Gadella TW Jr, Heidstra R, Scheres B, Blilou I. SCARECROW-LIKE23 and SCARECROW jointly specify endodermal cell fate but distinctly control SHORT-ROOT movement. Plant J. 2015 Sep 28. doi: 10.1111/tpj.13038. PubMed PMID: 26415082.
Long Y, Smet W, Cruz-Ramírez A, Castelijns B, de Jonge W, Mähönen AP, Bouchet BP, Perez GS, Akhmanova A, Scheres B, Blilou I. Arabidopsis BIRD Zinc Finger Proteins Jointly Stabilize Tissue Boundaries by Confining the Cell Fate Regulator SHORT-ROOT and Contributing to Fate Specification. Plant Cell. 2015 Apr;27(4):1185-99. doi: 10.1105/tpc.114.132407. Epub 2015 Mar 31. PubMed PMID: 25829440.
The I, Ruijtenberg S, Bouchet BP, Cristobal A, Prinsen MB, van Mourik T, Koreth J, Xu H, Heck AJ, Akhmanova A, Cuppen E, Boxem M, Muñoz J, van den Heuvel S. Rb and FZR1/Cdh1 determine CDK4/6-cyclin D requirement in C. elegans and human cancer cells. Nat Commun. 2015 Jan 6;6:5906. doi: 10.1038/ncomms6906. PubMed PMID: 25562820; PubMed Central PMCID: PMC4354291.
2014
Schlager MA, Serra-Marques A, Grigoriev I, Gumy LF, Esteves da Silva M, Wulf PS, Akhmanova A, Hoogenraad CC. Bicaudal d family adaptor proteins control the velocity of Dynein-based movements. Cell Rep. 2014 Sep 11;8(5):1248-56. doi: 10.1016/j.celrep.2014.07.052. Epub 2014 Aug 28. PubMed PMID: 25176647.
Preciado López M, Huber F, Grigoriev I, Steinmetz MO, Akhmanova A, Koenderink GH, Dogterom M. Actin-microtubule coordination at growing microtubule ends. Nat Commun. 2014 Aug 27;5:4778. doi: 10.1038/ncomms5778. PubMed PMID: 25159196.
Van Battum EY, Gunput RA, Lemstra S, Groen EJ, Yu KL, Adolfs Y, Zhou Y, Hoogenraad CC, Yoshida Y, Schachner M, Akhmanova A, Pasterkamp RJ. The intracellular redox protein MICAL-1 regulates the development of hippocampal mossy fibre connections. Nat Commun. 2014 Jul 10;5:4317. doi: 10.1038/ncomms5317. PubMed PMID: 25007825.
Yau KW, van Beuningen SF, Cunha-Ferreira I, Cloin BM, van Battum EY, Will L, Schätzle P, Tas RP, van Krugten J, Katrukha EA, Jiang K, Wulf PS, Mikhaylova M, Harterink M, Pasterkamp RJ, Akhmanova A, Kapitein LC, Hoogenraad CC. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014 Jun 4;82(5):1058-73. doi: 10.1016/j.neuron.2014.04.019. PubMed PMID: 24908486.
Jaarsma D, van den Berg R, Wulf PS, van Erp S, Keijzer N, Schlager MA, de Graaff E, De Zeeuw CI, Pasterkamp RJ, Akhmanova A, Hoogenraad CC. A role for Bicaudal-D2 in radial cerebellar granule cell migration. Nat Commun. 2014 Mar 11;5:3411. doi: 10.1038/ncomms4411. PubMed PMID: 24614806.
Jiang K, Hua S, Mohan R, Grigoriev I, Yau KW, Liu Q, Katrukha EA, Altelaar AF, Heck AJ, Hoogenraad CC, Akhmanova A. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev Cell. 2014 Feb 10;28(3):295-309. doi: 10.1016/j.devcel.2014.01.001. Epub 2014 Jan 30. PubMed PMID: 24486153.
Doodhi H, Katrukha EA, Kapitein LC, Akhmanova A. Mechanical and geometrical constraints control kinesin-based microtubule guidance. Curr Biol. 2014 Feb 3;24(3):322-8. doi: 10.1016/j.cub.2014.01.005. Epub 2014 Jan 23. PubMed PMID: 24462000.
Preciado López M, Huber F, Grigoriev I, Steinmetz MO, Akhmanova A, Dogterom M, Koenderink GH. In vitro reconstitution of dynamic microtubules interacting with actin filament networks. Methods Enzymol. 2014;540:301-20. doi: 10.1016/B978-0-12-397924-7.00017-0. PubMed PMID: 24630114.
2013
Shahbazi MN, Megias D, Epifano C, Akhmanova A, Gundersen GG, Fuchs E, and Perez-Moreno M. CLASP2 interacts with p120-catenin and governs microtubule dynamics at adherens junctions. J Cell Biol. 2013 Dec 23, 203(6): 1043-61. PMID: 24368809.
van der Vaart B, van Riel WE, Doodhi H, Kevenaar JT, Katrukha EA, Gumy L, Bouchet BP, Grigoriev I, Spangler SA, Yu KL, Wulf PS, Wu J, Lansbergen G, van Battum EY, Pasterkamp RJ, Mimori-Kiyosue Y, Demmers J, Olieric N, Maly IV, Hoogenraad CC, and Akhmanova A. CFEOM1-associated kinesin KIF21A is a cortical microtubule growth inhibitor. Dev Cell. 2013 Oct 28, 27(2): 145-60. PMID: 24120883.
Hu DJ, Baffet AD, Nayak T, Akhmanova A, Doye V, and Vallee RB. Dynein recruitment to nuclear pores activates apical nuclear migration and mitotic entry in brain progenitor cells. Cell. 2013 Sep 12, 154(6): 1300-13. PMID: 24034252.
van Spronsen M, van Battum EY, Kuijpers M, Vangoor VR, Rietman ML, Pothof J, Gumy LF, van Ijcken WF, Akhmanova A, Pasterkamp RJ, and Hoogenraad CC. Developmental and activity-dependent miRNA expression profiling in primary hippocampal neuron cultures. PLoS One. 2013, 8(10): e74907. PMID: 24098357.
Sen I, Veprintsev D, Akhmanova A, and Steinmetz MO. End binding proteins are obligatory dimers. PLoS One. 2013, 8(9): e74448. PMID: 24040250.
Berends CW, Munoz J, Portegijs V, Schmidt R, Grigoriev I, Boxem M, Akhmanova A, Heck AJ, and van den Heuvel S. F-actin asymmetry and the endoplasmic reticulum-associated TCC-1 protein contribute to stereotypic spindle movements in the Caenorhabditis elegans embryo. Mol Biol Cell. 2013 Jul, 24(14): 2201-15. PMID: 23699393.
Kuijpers M, Yu KL, Teuling E, Akhmanova A, Jaarsma D, and Hoogenraad CC. The ALS8 protein VAPB interacts with the ER-Golgi recycling protein YIF1A and regulates membrane delivery into dendrites. EMBO J. 2013 Jul 17, 32(14): 2056-72. PMID: 23736259.
Bulgakova NA, Grigoriev I, Yap AS, Akhmanova A, and Brown NH. Dynamic microtubules produce an asymmetric E-cadherin-Bazooka complex to maintain segment boundaries. J Cell Biol. 2013 Jun 10, 201(6): 887-901. PMID: 23751496.
Ferreira JG, Pereira AJ, Akhmanova A, and Maiato H. Aurora B spatially regulates EB3 phosphorylation to coordinate daughter cell adhesion with cytokinesis. J Cell Biol. 2013 May 27, 201(5): 709-24. PMID: 23712260.
Kapitein LC, van Bergeijk P, Lipka J, Keijzer N, Wulf PS, Katrukha EA, Akhmanova A, and Hoogenraad CC. Myosin-V opposes microtubule-based cargo transport and drives directional motility on cortical actin. Curr Biol. 2013 May 6, 23(9): 828-34. PMID: 23602478.
Molina A, Velot L, Ghouinem L, Abdelkarim M, Bouchet BP, Luissint AC, Bouhlel I, Morel M, Sapharikas E, Di Tommaso A, Honore S, Braguer D, Gruel N, Vincent-Salomon A, Delattre O, Sigal-Zafrani B, Andre F, Terris B, Akhmanova A, Di Benedetto M, Nahmias C, and Rodrigues-Ferreira S. ATIP3, a novel prognostic marker of breast cancer patient survival, limits cancer cell migration and slows metastatic progression by regulating microtubule dynamics. Cancer Res. 2013 May 1, 73(9): 2905-15. PMID: 23396587.
Jeffery JM, Grigoriev I, Poser I, van der Horst A, Hamilton N, Waterhouse N, Bleier J, Subramaniam VN, Maly IV, Akhmanova A, and Khanna KK. Centrobin regulates centrosome function in interphase cells by limiting pericentriolar matrix recruitment. Cell Cycle. 2013 Mar 15, 12(6): 899-906. PMID: 23442802.
van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, Jaarsma D, Gerritsen HC, Akhmanova A, and Hoogenraad CC. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 2013 Feb 6, 77(3): 485-502. PMID: 23395375.
2012
van der Vaart B, Franker MA, Kuijpers M, Hua S, Bouchet BP, Jiang K, Grigoriev I, Hoogenraad CC, and Akhmanova A. Microtubule plus-end tracking proteins SLAIN1/2 and ch-TOG promote axonal development. J Neurosci. 2012 Oct 17, 32(42): 14722-8. PMID: 23077057.
Splinter D, Razafsky DS, Schlager MA, Serra-Marques A, Grigoriev I, Demmers J, Keijzer N, Jiang K, Poser I, Hyman AA, Hoogenraad CC, King SJ, Akhmanova A.BICD2, dynactin and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol Biol Cell. 2012 Sep 5.PMID:22956769
Jiang K, Toedt G, Montenegro Gouveia S, Davey NE, Hua S, van der Vaart B, Grigoriev I, Larsen J, Pedersen LB, Bezstarosti K, Lince-Faria M, Demmers J, Steinmetz MO, Gibson TJ, Akhmanova A. A Proteome-wide Screen for Mammalian SxIP Motif-Containing Microtubule Plus-End Tracking Proteins. Curr Biol. 2012 Oct 9;22(19):1800-7. PMID:22885064
Louwen R, Nieuwenhuis EE, van Marrewijk L, Horst-Kreft D, de Ruiter L, Heikema AP, van Wamel WJ, Wagenaar JA, Endtz HP, Samsom J, van Baarlen P, Akhmanova A, van Belkum A. Campylobacter jejuni translocation across intestinal epithelial cells is facilitated by ganglioside-like lipooligosaccharide structures. Infect Immun. 2012 Sep;80(9):3307-18. PMID:22778098
Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012 Aug;14(8):818-28. PMID:22750944
Lui-Roberts WW, Stinchcombe JC, Ritter AT, Akhmanova A, Karakesisoglou I, Griffiths GM. Cytotoxic T lymphocyte effector function is independent of nucleus-centrosome dissociation. Eur J Immunol. 2012 Aug;42(8):2132-41. PMID: 22736282
Buey RM, Sen I, Kortt O, Mohan R, Gfeller D, Veprintsev D, Kretzschmar I, Scheuermann J, Neri D, Zoete V, Michielin O, de Pereda JM, Akhmanova A, Volkmer R, Steinmetz MO. Sequence determinants of a microtubule tip localization signal (MtLS). J Biol Chem. 2012 Aug 17;287(34):28227-42. PMID: 22696216
Pagano A, Honoré S, Mohan R, Berges R, Akhmanova A, Braguer D.Epothilone B inhibits migration of glioblastoma cells by inducing microtubule catastrophes and affecting EB1 accumulation at microtubule plus ends. Biochem Pharmacol. 2012 Aug 15;84(4):432-43. PMID:22634050
Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012 Mar 5;196(5):641-52. PMID: 22391038
2011
Lomakin AJ, Kraikivski P, Semenova I, Ikeda K, Zaliapin I, Tirnauer JS, Akhmanova A, Rodionov V. Stimulation of the CLIP-170–dependent capture of membrane organelles by microtubules through fine tuning of microtubule assembly dynamics. Mol Biol Cell. 2011 Nov;22(21):4029-37. Epub 2011 Aug 31. PMID: 21880898
Tanenbaum ME, Macurek L, van der Vaart B, Galli M, Akhmanova A, Medema RH. A complex of Kif18b and MCAK promotes microtubule depolymerization and is negatively regulated by Aurora kinases.Curr Biol. 2011 Aug 23;21(16):1356-65. Epub 2011 Aug 4. PMID: 21820309
Kovacs EM, Verma S, Ali RG, Ratheesh A, Hamilton NA, Akhmanova A, Yap AS. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat Cell Biol. 2011 Jul 24. doi: 10.1038/ncb2290. PMID: 21785420
van der Vaart B, Manatschal C, Grigoriev I, Olieric V, Gouveia SM, Bjelic S, Demmers J, Vorobjev I, Hoogenraad CC, Steinmetz MO, Akhmanova A. SLAIN2 links microtubule plus end-tracking proteins and controls microtubule growth in interphase. J Cell Biol. 2011 Jun 13;193(6):1083-99. Epub 2011 Jun 6.PMID: 21646404
Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E, Demmers J, Peränen J, Pasterkamp RJ, van der Sluijs P, Hoogenraad CC, Akhmanova A.Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr Biol. 2011 Jun 7;21(11):967-74. Epub 2011 May 19. PMID: 21596566
Schrøder JM, Larsen J, Komarova Y, Akhmanova A, Thorsteinsson RI, Grigoriev I, Manguso R, Christensen ST, Pedersen SF, Geimer S, Pedersen LB. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J Cell Sci. 2011 Aug 1;124(Pt 15):2539-51.PMID:21768326
Buey RM, Mohan R, Leslie K, Walzthoeni T, Missimer JH, Menzel A, Bjelic S, Bargsten K, Grigoriev I, Smal I, Meijering E, Aebersold R, Akhmanova A, Steinmetz MO. Insights into EB structure and the role of its C-terminal domain in discriminating microtubule tips from lattice. Mol Biol Cell. 2011 Jul 7.PMID: 21737692
Kapitein LC, Yau KW, Gouveia SM, van der Zwan WA, Wulf PS, Keijzer N, Demmers J, Jaworski J, Akhmanova A, Hoogenraad CC.NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci. 2011 Jun 1;31(22):8194-209. PMID:21632941
Spangler SA, Jaarsma D, de Graaff E, Wulf PS, Akhmanova A, Hoogenraad CC. Differential expression of liprin-α family proteins in the brain suggests functional diversification. J Comp Neurol. 2011 May 25. doi: 10.1002/cne.22665. PMID:21618222
2010-2000
Montenegro Gouveia S, Leslie K, Kapitein LC, Buey RM, Grigoriev I, Wagenbach M, Smal I, Meijering E, Hoogenraad CC, Wordeman L, Steinmetz MO, Akhmanova A. In vitro reconstitution of the functional interplay between MCAK and EB3 at microtubule plus ends. Curr Biol. 2010 Oct 12;20(19):1717-22. Epub 2010 Sep 16.PMID:20850319
Splinter D, Tanenbaum ME, Lindqvist A, Jaarsma D, Flotho A, Yu KL, Grigoriev I, Engelsma D, Haasdijk ED, Keijzer N, Demmers J, Fornerod M, Melchior F, Hoogenraad CC, Medema RH, Akhmanova A. Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry.PLoS Biol. 2010 Apr 6;8(4):e1000350.PMID: 20386726
Lee HS, Komarova YA, Nadezhdina ES, Anjum R, Peloquin JG, Schober JM, Danciu O, van Haren J, Galjart N, Gygi SP, Akhmanova A, Borisy GG.Phosphorylation controls autoinhibition of cytoplasmic linker protein-170.Mol Biol Cell. 2010 Aug 1;21(15):2661-73. Epub 2010 Jun 2. PMID:20519438
Hotta A, Kawakatsu T, Nakatani T, Sato T, Matsui C, Sukezane T, Akagi T, Hamaji T, Grigoriev I, Akhmanova A, Takai Y, Mimori-Kiyosue Y. Laminin-based cell adhesion anchors microtubule plus ends to the epithelial cell basal cortex through LL5alpha/beta.J Cell Biol. 2010 May 31;189(5):901-17.PMID:20513769
Schlager MA, Kapitein LC, Grigoriev I, Burzynski GM, Wulf PS, Keijzer N, de Graaff E, Fukuda M, Shepherd IT, Akhmanova A, Hoogenraad CC. Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis. EMBO J. 2010 May 19;29(10):1637-51. Epub 2010 Apr 1. PMID:20360680
Smal I, Grigoriev I, Akhmanova A, Niessen WJ, Meijering E.Microtubule dynamics analysis using kymographs and variable-rate particle filters.IEEE Trans Image Process. 2010 Jul;19(7):1861-76. Epub 2010 Mar 11.PMID:20227980
De Groot CO, Jelesarov I, Damberger FF, Bjelic S, Schaerer MA, Bhavesh NS, Grigoriev I, Buey RM, Wuthrich K, Capitani G, Akhmanova A, Steinmetz MO. Molecular insights into mammalian end binding protein heterodimerization. J Biol Chem. 2010 Feb 19;285(8):5802-14. Epub 2009 Dec 12. PMID: 20008324 PubMed
2009
Smal I, Grigoriev I, Akhmanova A, Niessen WJ, Meijering E. Accurate estimation of microtubule dynamics using kymographs and variable-rate particle filters. Conf Proc IEEE Eng Med Biol Soc. 2009;1:1012-5. PMID: 19963986 PubMed
Lomakin AJ, Semenova I, Zaliapin I, Kraikivski P, Nadezhdina E, Slepchenko BM, Akhmanova A, Rodionov V. CLIP-170-dependent capture of membrane organelles by microtubules initiates minus-end directed transport. Dev Cell. 2009 Sep;17(3):323-33. PMID: 19758557 PubMed
Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wüthrich K, Akhmanova A, Steinmetz MO. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009 Jul 23;138(2):366-76. PMID: 19632184 PubMed
Komarova Y, De Groot CO, Grigoriev I, Gouveia SM, Munteanu EL, Schober JM, Honnappa S, Buey RM, Hoogenraad CC, Dogterom M, Borisy GG, Steinmetz MO, Akhmanova A. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol. 2009 Mar 9;184(5):691-706. Epub 2009 Mar 2. PMID: 19255245 PubMed
Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, Hoogenraad CC. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009 Jan 15;61(1):85-100. PMID: 19146815 PubMed
2008
Teuling E, van Dis V, Wulf PS, Haasdijk ED, Akhmanova A, Hoogenraad CC, Jaarsma D. A novel mouse model with impaired dynein/dynactin function develops amyotrophic lateral sclerosis (ALS)-like features in motor neurons and improves lifespan in SOD1-ALS mice. Hum Mol Genet. 2008 Sep 15;17(18):2849-62. Epub 2008 Jun 25. PMID: 18579581 PubMed
Smal I, Meijering E, Draegestein K, Galjart N, Grigoriev I, Akhmanova A, van Royen ME, Houtsmuller AB, Niessen W. Multiple object tracking in molecular bioimaging by Rao-Blackwellized marginal particle filtering. Med Image Anal. 2008 Dec;12(6):764-77. Epub 2008 Mar 31. PMID: 18457985 [PubMed – indexed for MEDLINE]
Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrásek J, Seifertová D, Tejos R, Meisel LA, Zazímalová E, Gadella TW Jr, Stierhof YD, Ueda T, Oiwa K, Akhmanova A, Brock R, Spang A, Friml J. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci U S A. 2008 Mar 18;105(11):4489-94. Epub 2008 Mar 12. PMID: 18337510 PubMed
Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW Jr, Hoogenraad CC, Akhmanova A. STIM1 Is a MT-Plus-End-Tracking Protein Involved in Remodeling of the ER. Curr Biol. 2008 Feb 12;18(3):177-82. Epub 2008 Jan 31. PMID: 18249114 PubMed
Dragestein KA, van Cappellen WA, van Haren J, Tsibidis GD, Akhmanova A, Knoch TA, Grosveld F, Galjart N. Dynamic behavior of GFP-CLIP-170 reveals fast protein turnover on microtubule plus ends. J Cell Biol. 2008 Feb 25;180(4):729-37. Epub 2008 Feb 18. PMID: 18283108 PubMed
2007
Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, Akhmanova A. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007 Aug;13(2):305-14. PMID: 17681140 PubMed
Weisbrich A, Honnappa S, Jaussi R, Okhrimenko O, Frey D, Jelesarov I, Akhmanova A, Steinmetz MO. Structure-function relationship of CAP-Gly domains. Nat Struct Mol Biol. 2007 Oct;14(10):959-67. Epub 2007 Sep 9. PMID: 17828277 PubMed
Teuling E, Ahmed S, Haasdijk E, Demmers J, Steinmetz MO, Akhmanova A, Jaarsma D, Hoogenraad CC. Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. J Neurosci. 2007 Sep 5;27(36):9801-15. PMID: 17804640 PubMed
Wanschers BF, van de Vorstenbosch R, Schlager MA, Splinter D, Akhmanova A, Hoogenraad CC, Wieringa B, Fransen JA. A role for the Rab6B Bicaudal-D1 interaction in retrograde transport in neuronal cells. Exp Cell Res. 2007 Oct 1;313(16):3408-20. Epub 2007 Jul 17. PMID: 17707369 PubMed
Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, Yates JR 3rd, Maiato H, Khodjakov A, Akhmanova A, Kaverina I. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007 Jun;12(6):917-30. PMID: 17543864 PubMed
Schober JM, Komarova YA, Chaga OY, Akhmanova A, Borisy GG. Microtubule-targeting-dependent reorganization of filopodia. J Cell Sci. 2007 Apr 1;120(Pt 7):1235-44. Epub 2007 Mar 13. PMID: 17356063 PubMed
Tsvetkov AS, Samsonov A, Akhmanova A, Galjart N, Popov SV. Microtubule-binding proteins CLASP1 and CLASP2 interact with actin filaments. Cell Motil Cytoskeleton. 2007 Jul;64(7):519-30. PMID: 17342765 PubMed
2006
Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, Ohtsuka T, Higa S, Kitajima I, Demmers J, Galjart N, Houtsmuller AB, Grosveld F, Akhmanova A. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell. 2006 Jul;11(1):21-32. PMID: 16824950 PubMed
Drabek K, van Ham M, Stepanova T, Draegestein K, van Horssen R, Sayas CL, Akhmanova A, Ten Hagen T, Smits R, Fodde R, Grosveld F, Galjart N. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol. 2006 Nov 21;16(22):2259-64. PMID: 17113391 PubMed
Mimori-Kiyosue Y, Grigoriev I, Sasaki H, Matsui C, Akhmanova A, Tsukita S, Vorobjev I. Mammalian CLASPs are required for mitotic spindle organization and kinetochore alignment. Genes Cells. 2006 Aug;11(8):845-57. PMID: 16866869 PubMed
Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Parton RG, Yap AS. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci. 2006 May 1;119(Pt 9):1801-11. Epub 2006 Apr 11. PMID: 16608875 PubMed
2005
Akhmanova A, Mausset-Bonnefont AL, van Cappellen W, Keijzer N, Hoogenraad CC, Stepanova T, Drabek K, van der Wees J, Mommaas M, Onderwater J, van der Meulen H, Tanenbaum ME, Medema RH, Hoogerbrugge J, Vreeburg J, Uringa EJ, Grootegoed JA, Grosveld F, Galjart N. The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev. 2005 Oct 15;19(20):2501-15. PMID: 16230537 PubMed
Komarova Y, Lansbergen G, Galjart N, Grosveld F, Borisy GG, Akhmanova A. EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol Biol Cell. 2005 Nov;16(11):5334-45. Epub 2005 Sep 7. PMID: 16148041 PubMed
Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, Akhmanova A. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005 Jan 3;168(1):141-53. PMID: 15631994 PubMed
2004
Lansbergen G, Komarova Y, Modesti M, Wyman C, Hoogenraad CC, Goodson HV, Lemaitre RP, Drechsel DN, van Munster E, Gadella TW Jr, Grosveld F, Galjart N, Borisy GG, Akhmanova A. Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J Cell Biol. 2004 Sep 27;166(7):1003-14. Epub 2004 Sep 20. PMID: 15381688 PubMed
2003
Hoogenraad CC, Wulf P, Schiefermeier N, Stepanova T, Galjart N, Small JV, Grosveld F, de Zeeuw CI, Akhmanova A. Bicaudal D induces selective dynein-mediated microtubule minus end-directed transport. EMBO J. 2003 Nov 17;22(22):6004-15. PMID: 14609947 PubMed
Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci. 2003 Apr 1;23(7):2655-64. PMID: 12684451 PubMed
2002
Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, Barnekow A, Hoogenraad CC. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002 Dec;4(12):986-92. Erratum in: Nat Cell Biol. 2003 Jan;5(1):84. PMID: 12447383 PubMed
Komarova YA, Akhmanova AS, Kojima S, Galjart N, Borisy GG. Cytoplasmic linker proteins promote microtubule rescue in vivo. J Cell Biol. 2002 Nov 25;159(4):589-99. PMID: 12446741 PubMed
Hoogenraad CC, Koekkoek B, Akhmanova A, Krugers H, Dortland B, Miedema M, van Alphen A, Kistler WM, Jaegle M, Koutsourakis M, Van Camp N, Verhoye M, van der Linden A, Kaverina I, Grosveld F, De Zeeuw CI, Galjart N. Targeted mutation of Cyln2 in the Williams syndrome critical region links CLIP-115 haploinsufficiency to neurodevelopmental abnormalities in mice. Nat Genet. 2002 Sep;32(1):116 27. Epub 2002 Aug 26. Erratum in: Nat Genet 2002 Oct;32(2):331. PMID: 12195424 PubMed
Coquelle FM, Caspi M, Cordelieres FP, Dompierre JP, Dujardin DL, Koifman C, Martin P, Hoogenraad CC, Akhmanova A, Galjart N, De Mey JR, Reiner O. LIS1, CLIP-170’s key to the dynein/dynactin pathway. Mol Cell Biol. 2002 May;22(9):3089-102. PMID: 11940666 PubMed
2001
Hoogenraad CC, Akhmanova A, Howell SA, Dortland BR, De Zeeuw CI, Willemsen R, Visser P, Grosveld F, Galjart N. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 2001 Aug 1;20(15):4041-54. PMID: 11483508 PubMed
Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, Galjart N. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001 Mar 23;104(6):923-35. PMID: 11290329 PubMed
2000
Akhmanova A, Verkerk T, Langeveld A, Grosveld F, Galjart N. Characterisation of transcriptionally active and inactive chromatin domains in neurons. J Cell Sci. 2000 Dec;113 Pt 24:4463-74. PMID: 11082040 PubMed
Hoogenraad CC, Akhmanova A, Grosveld F, De Zeeuw CI, Galjart N. Functional analysis of CLIP-115 and its binding to microtubules. J Cell Sci. 2000 Jun;113 ( Pt 12):2285-97. PMID: 10825300 PubMed
Reviews
2024
Thery M, Akhmanova A. Confined migration: Microtubules control the cell rear. Curr Biol. 2024 Aug 5;34(15):R728-R731. doi: 10.1016/j.cub.2024.06.045. PMID: 39106829.
Volkov VA, Akhmanova A. Phase separation on microtubules: from droplet formation to cellular function? Trends Cell Biol. 2024 Jan;34(1):18-30. doi: 10.1016/j.tcb.2023.06.004. Epub 2023 Jul 13. PMID: 37453878.
2023
2022
Eisen MB, Akhmanova A, Behrens TE, Diedrichsen J, Harper DM, Iordanova MD, Weigel D, Zaidi M. Peer review without gatekeeping. Elife. 2022 Oct 20;11:e83889. doi: 10.7554/eLife.83889. PMID: 36263932; PMCID: PMC9584602.
Akhmanova A, Kapitein LC. Mechanisms of microtubule organization in differentiated animal cells. Nat Rev Mol Cell Biol. 2022 Aug;23(8):541-558. doi: 10.1038/s41580-022-00473-y. Epub 2022 Apr 5. PMID: 35383336.
2021
Zaidi M, Harper DM, Akhmanova A, Weigel D, Behrens TE, Eisen MB. Rigorous review and editorial oversight of clinical preprints. Elife. 2021 Jun 16;10:e67528. doi: 10.7554/eLife.67528. PMID: 34130793; PMCID: PMC8208811.
2020
Meiring JCM, Shneyer BI, and Akhmanova A. Generation and regulation of microtubule network asymmetry to drive cell polarity. Curr Opin Cell Biol 2020, 62: 86-95.
Meiring JCM and Akhmanova A. Microtubules keep large cells in shape. J Cell Biol 2020, 219(6).
2019
Akhmanova A and Steinmetz MO. Microtubule minus-end regulation at a glance. J Cell Sci 2019, 132(11).
2018
Martin M and Akhmanova A. Coming into Focus: Mechanisms of Microtubule Minus-End Organization. Trends Cell Biol 2018, 28(7): 574-588.
Aher A and Akhmanova A. Tipping microtubule dynamics, one protofilament at a time. Curr Opin Cell Biol 2018, 50: 86-93.
Akhmanova A. Strengthening Microtubules by Cuts that Heal. Dev Cell 2018, 47(4): 400-401.
Akhmanova A and Hoogenraad CC. More is not always better: hyperglutamylation leads to neurodegeneration. EMBO J 2018, 37(23): e101023.
2017
Akhmanova A and Maiato H. Closing the tubulin detyrosination cycle. Science, 2017; 358 (6369): 1381-1382
Bouchet BP and Akhmanova A. Microtubules in 3D cell motility. J Cell Sci, 2017; 130 (1): 39-50.
Noordstra I and Akhmanova A. Linking cortical microtubule attachment and exocytosis. F1000Res, 2017; 6: 469.
Wu J and Akhmanova A. Microtubule-Organizing Centers. Annu Rev Cell Dev Biol, 2017; 33: 51-75.
2016
Akhmanova A and van den Heuvel S. Tipping the spindle into the right position. J Cell Biol, 2016. 213(3): 293-5.
Hoogenraad CC and Akhmanova A. Bicaudal D Family of Motor Adaptors: Linking Dynein Motility to Cargo Binding. Trends Cell Biol, 2016. 26(5): 327-40.
van de Willige D, Hoogenraad CC, and Akhmanova A. Microtubule plus-end tracking proteins in neuronal development. Cell Mol Life Sci, 2016. 73(10): 2053-77.
2015
Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015 Dec;16(12):711-26. doi: 10.1038/nrm4084. Epub 2015 Nov 12. PubMed PMID: 26562752.
Akhmanova A, Hoogenraad CC. Microtubule minus-end-targeting proteins. Curr Biol. 2015 Feb 16;25(4):R162-71. doi: 10.1016/j.cub.2014.12.027. Review. PubMed PMID: 25689915.
2014
Pedersen LB, Akhmanova A. Kif7 keeps cilia tips in shape. Nat Cell Biol. 2014 Jul;16(7):623-5. doi: 10.1038/ncb2997. PubMed PMID: 24981634.
2013
Larsen J, Grigoriev I, Akhmanova A, and Pedersen LB. Analysis of microtubule plus-end-tracking proteins in cilia. Methods Enzymol. 2013, 524: 105-22. PMID: 23498737.
Akhmanova A and Stearns T. Cell architecture: putting the building blocks together. Curr Opin Cell Biol. 2013 Feb, 25(1): 3-5. PMID: 23279910
2011
Akhmanova A, Dogterom M.Kinesins lead aging microtubules to catastrophe. Cell. 2011 Nov 23;147(5):966-8. PMID:22118452
Tanenbaum ME, Medema RH, Akhmanova A. Regulation of localization and activity of the microtubule depolymerase MCAK. Bioarchitecture. 2011 Mar;1(2):80-87. PMID: 21866268
Akhmanova A, Steinmetz MO. Microtubule end binding: EBs sense the guanine nucleotide state. Curr Biol. 2011 Apr 26;21(8):R283-5. PMID:21514511
Yu KL, Keijzer N, Hoogenraad CC, Akhmanova A. Isolation of Novel +TIPs and Their Binding Partners Using Affinity Purification Techniques. Methods Mol Biol. 2011;777:293-316.PMID:21773937
Tanenbaum ME, Akhmanova A, Medema RH. Bi-directional transport of the nucleus by dynein and kinesin-1. Commun Integr Biol. 2011 Jan;4(1):21-5.PMID:21509171
Jiang K, Akhmanova A. Microtubule tip-interacting proteins: a view from both ends. Curr Opin Cell Biol. 2011 Feb;23(1):94-101. PMID: 20817499
2010
Hoogenraad CC, Akhmanova A. Dendritic spine plasticity: new regulatory roles of dynamic microtubules.Neuroscientist. 2010 Dec;16(6):650-61.PMID: 21239729
Gouveia SM, Akhmanova A. Cell and molecular biology of microtubule plus end tracking proteins: end binding proteins and their partners. Int Rev Cell Mol Biol. 2010;285:1-74. Review. PMID: 21035097
Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010 Oct 15;123(Pt 20):3415-9. Review. No abstract available. PMID: 20930136
Tanenbaum ME, Akhmanova A, Medema RH. Dynein at the nuclear envelope. EMBO Rep. 2010 Sep;11(9):649. PMID: 20805838
Grigoriev I, Akhmanova A. Microtubule dynamics at the cell cortex probed by TIRF microscopy. Methods Cell Biol. 2010;97:91-109. Review. PMID: 20719267
Akhmanova A, Hammer JA 3rd. Linking molecular motors to membrane cargo.Curr Opin Cell Biol. 2010 Aug;22(4):479-87. Epub 2010 May 11. Review. PMID: 20466533
2009
van der Vaart B, Akhmanova A, Straube A. Regulation of microtubule dynamic instability. Biochem Soc Trans. 2009 Oct;37(Pt 5):1007-13. Review. PMID: 19754441 PubMed
Stehbens SJ, Akhmanova A, Yap AS. Microtubules and cadherins: a neglected partnership. Front Biosci. 2009 Jan 1;14:3159-67. Review. PMID: 19273264 PubMed
Akhmanova A, Stehbens SJ, Yap AS. Touch, grasp, deliver and control: functional cross-talk between microtubules and cell adhesions. Traffic. 2009 Mar;10(3):268-74. Epub 2009 Jan 17. Review. PMID: 19175539 PubMed
2008
Akhmanova A, Yap AS. Organizing junctions at the cell-cell interface. Cell. 2008 Nov 28;135(5):791-3. PMID: 19041742 PubMed
Steinmetz MO, Akhmanova A. Capturing protein tails by CAP-Gly domains. Trends Biochem Sci. 2008 Nov;33(11):535-45. Epub 2008 Oct 4. Review. PMID: 18835717 PubMed
Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008 Mar 5; [Epub ahead of print] PMID: 18322465 PubMed
2007
Jaworski J, Hoogenraad CC, Akhmanova A. Microtubule plus-end tracking proteins in differentiated mammalian cells. Int J Biochem Cell Biol. 2007 Oct 22; [Epub ahead of print] PMID: 18023603 PubMed
2006
Lansbergen G, Akhmanova A. Microtubule plus end: a hub of cellular activities. Traffic. 2006 May;7(5):499-507. PMID: 16643273 PubMed
2005
Akhmanova A, Hoogenraad CC. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol. 2005 Feb;17(1):47-54. Review. PMID: 15661518 PubMed
2004
Akhmanova A, Severin F. Thirteen is the lucky number for doublecortin. Dev Cell. 2004 Jul;7(1):5-6. Review. PMID: 15239948 PubMed
Hoogenraad CC, Akhmanova A, Galjart N, De Zeeuw CI. LIMK1 and CLIP-115: linking cytoskeletal defects to Williams syndrome. Bioessays. 2004 Feb;26(2):141-50. Review. PMID: 14745832 PubMed

