Splinter D, Razafsky DS, Schlager MA, Serra-Marques A, Grigoriev I, Demmers J, Keijzer N, Jiang K, Poser I, Hyman AA, Hoogenraad CC, King SJ, Akhmanova A. BICD2, dynactin and LIS1 cooperate in regulating dynein recruitment to cellular structures.
Mol Biol Cell. 2012 Sep 5.PMID:22956769
Cytoplasmic dynein is the major microtubule minus-end directed cellular motor. Most dynein activities require dynactin, but the mechanisms regulating cargo-dependent dynein-dynactin interaction are poorly understood. In this study, we focus on dynein-dynactin recruitment to cargo by the conserved motor adaptor BICD2. We show that dynein and dynactin depend on each other for BICD2-mediated targeting to cargo, and that BICD2 N-terminus (BICD2-N) strongly promotes stable interaction between dynein and dynactin both in vitro and in vivo. Direct visualization of dynein in live cells indicates that by itself the triple BICD2-N-dynein-dynactin complex is unable to interact with either cargo or microtubules. However, tethering of BICD2-N to different membranes promotes their microtubule minus-end directed motility. We further show that LIS1 is required for dynein-mediated transport induced by membrane tethering of BICD2-N, and that LIS1 contributes to dynein accumulation at microtubule plus ends and BICD2-positive cellular structures. Our results demonstrate that dynein recruitment to cargo requires concerted action of multiple dynein cofactors.
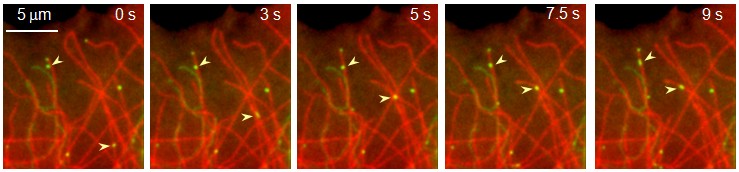
Movement of Rab6 vesicles (green) along microtubules (red).
Tethering of BICD2-N promotes microtubule minus end directed vesicle runs without affecting their velocity.

