Therapeutic stem cells expressing variants of EGFR-specific nanobodies have antitumor effects. Van de Water JA, Bagci-Onder T, Agarwal AS, Wakimoto H, Roovers RC, Zhu Y, Kasmieh R, Bhere D, Van Bergen En Henegouwen PMP, Shah K. Proc Natl Acad Sci U S A. 2012 Sep 25. Epub ahead of print, PMID: 23012408
The deregulation of the epidermal growth factor receptor (EGFR) has a significant role in the progression of tumors. Despite the development of a number of EGFR-targeting agents that can arrest tumor growth, their success in the clinic is limited in several tumor types, particularly in the highly malignant glioblastoma multiforme (GBM).
In this study, we generated and characterized EGFR-specific nanobodies (ENb) and imageable and proapoptotic ENb immunoconjugates released from stem cells (SC) to ultimately develop a unique EGFR-targeted therapy for GBM. We show that ENbs released from SCs specifically localize to tumors, inhibit EGFR signaling resulting in reduced GBM growth and invasiveness in vitro and in vivo in both established and primary GBM cell lines. We also show that ENb primes GBM cells for proapoptotic tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Furthermore, SC-delivered immunoconjugates of ENb and TRAIL target a wide spectrum of GBM cell types with varying degrees of TRAIL resistance and significantly reduce GBM growth and invasion in both established and primary invasive GBM in mice.
This study demonstrates the efficacy of Stem cell-based EGFR targeted therapy in glioblastoma multiforme and provides a unique approach with clinical implications.
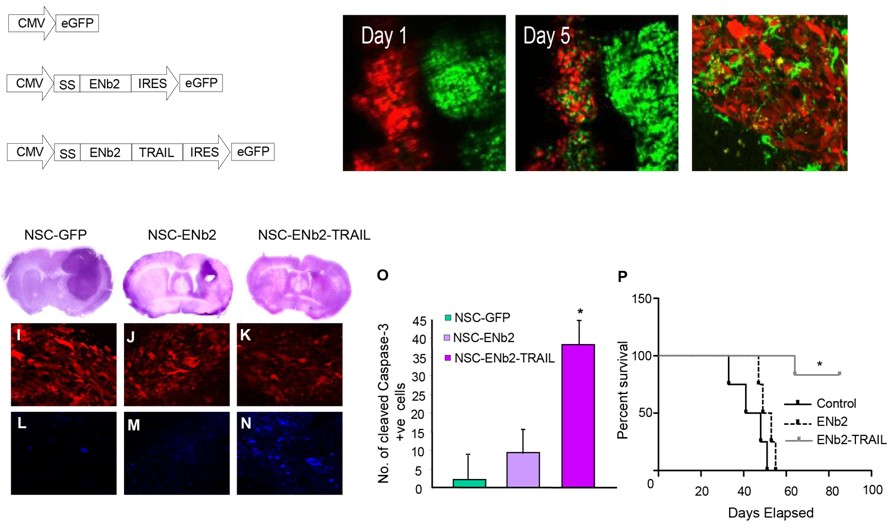
Three constructs, eGFP, anti-EGFR nanobodies (ENb2) and ENb2 fused to the apoptosis inducer TRAIL were expressed in Neural Stem Cells (green) and implanted next to highly malignant glioblastoma multifirme cells (red). NSC cells moved into the tumor and produced the constructs locally. Histochemical analysis of brain slices showed decrease in tumor volumes, induction of caspase activity and 80% of the mice treated with NSC-ENb2-TRAIL were alive 80 d after treatment.

