SLAIN2 links microtubule plus-end tracking proteins and controls microtubule dynamics in a cell-cycle dependent manner. Babet van der Vaart, Cristina Manatschal, Ilya Grigoriev, Vincent Olieric, Susana Montenegro Gouveia, Saša Bjelić, Jeroen Demmers, Ivan Vorobjev, Casper C. Hoogenraad, Michel O. Steinmetz and Anna Akhmanova. The Journal of Cell Biology 2011 Jun 13;193(6):1083-99. Epub 2011 Jun 6. PMID: 21646404
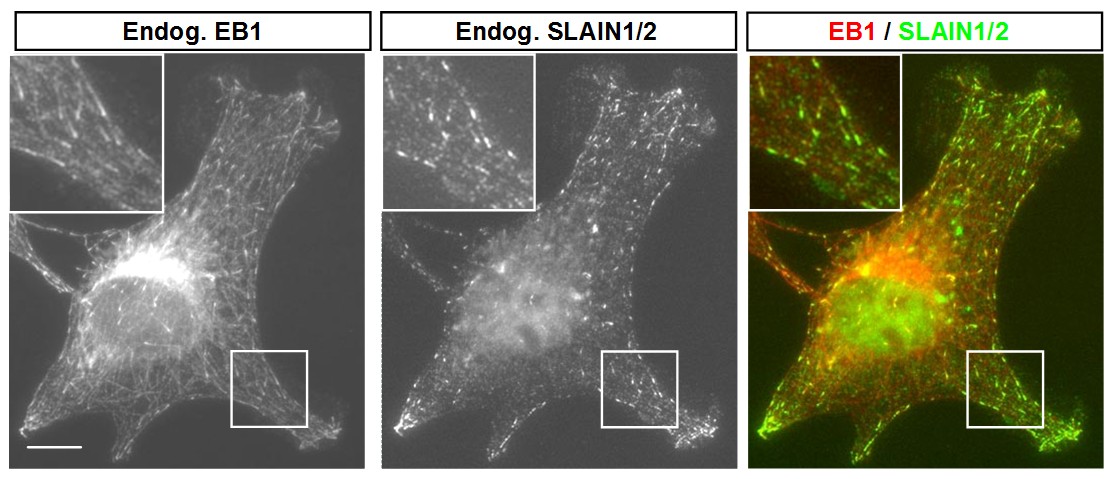
An important group of microtubule regulators known as plus-end tracking proteins (+TIPs) associate specifically with the growing microtubule ends. One of the most conserved +TIP families are the End Binding proteins (EBs). To isolate new +TIPs, we exploited the fact that the majority of known plus-end binding proteins associate directly with EBs. Using pull-down assays combined with mass spectrometry we identified the vertebrate protein SLAIN2 as a novel EB partner. Plus-end tracking behaviour of SLAIN2 depends on EBs and is mediated by SLAIN2 C-terminus. This region also interacts with two other major +TIP families the CLASPs and CLIPs. The latter interaction is critically dependent on two C-terminal residues of SLAIN2; a tryptophan and a tyrosine.
Through its N-terminus, SLAIN2 interacts with ch-TOG, the mammalian homologue of the microtubule polymerase XMAP215. SLAIN2 links ch-TOG to EBs and promotes ch-TOG association with growing microtubule plus-ends in cells. The depletion of SLAIN2 and ch-TOG from interphase cells results in similar phenotypes: catastrophe rate is strongly increased and persistent microtubule growth is inhibited. Surprisingly, whereas ch-TOG knockdown causes severe mitotic defects, this is not the case in SLAIN2-depleted cells. During mitosis, SLAIN2 is hyperphosphorylated, and its association with EBs and ch-TOG is disrupted. This effect is at least partially due the activity of the mitotic kinase CDK1.
Taken together, our study identifies SLAIN2 as an important part of a complex microtubule plus-end targeting mechanism and reveals the function of SLAIN2 in the cell-cycle regulated control of microtubule dynamics.






 Anna Akhmanova:
Cellular Dynamics
Anna Akhmanova:
Cellular Dynamics Lukas Kapitein: Biophysics
Lukas Kapitein: Biophysics Florian Berger:
Theoretical Biophysics
Florian Berger:
Theoretical Biophysics Agathe Chaigne:
Cell division dynamics
Agathe Chaigne:
Cell division dynamics Ginny Farías:
Neuronal Organelle Dynamics
Ginny Farías:
Neuronal Organelle Dynamics Martin Harterink:
C. elegans neurobiology
Martin Harterink:
C. elegans neurobiology Casper Hoogenraad: Molecular Neuroscience
Casper Hoogenraad: Molecular Neuroscience Maarten Kole:
Axonal Signalling
Maarten Kole:
Axonal Signalling Harold MacGillavry:
Synapse organization
Harold MacGillavry:
Synapse organization Sabrina Oliveira:
Molecular Targeted Therapies
Sabrina Oliveira:
Molecular Targeted Therapies Ihor Smal: Image Analysis, Smart Microscopy and AI
Ihor Smal: Image Analysis, Smart Microscopy and AI Frederik Verweij:
Extracellular Vesicle Biology
Frederik Verweij:
Extracellular Vesicle Biology