Benoit RM, Frey D, Hilbert M, Kevenaar JT, Wieser MM, Stirnimann CU, McMillan D, Ceska T, Lebon F, Jaussi R, Steinmetz MO, Schertler GFX, Hoogenraad CC, Capitani G and Kammerer RA, Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A, Nature, 2013
Botulinum neurotoxin A (BoNT/A) belongs to the most dangerous class of bioweapons. Despite this, BoNT/A is used to treat a wide range of common medical conditions such as migraines and a variety of ocular motility and movement disorders. BoNT/A is probably best known for its use as an antiwrinkle agent in cosmetic applications (including Botox and Dysport). BoNT/A application causes long-lasting flaccid paralysis of muscles through inhibiting the release of the neurotransmitter acetylcholine by cleaving synaptosomal-associated protein 25 (SNAP-25) within presynaptic nerve terminals4. Two types of BoNT/A receptor have been identified, both of which are required for BoNT/A toxicity and are therefore likely to cooperate with each other: gangliosides and members of the synaptic vesicle glycoprotein 2 (SV2) family, which are putative transporter proteins that are predicted to have 12 transmembrane domains, associate with the receptor-binding domain of the toxin. In SV2 proteins, the BoNT/A-binding site has been mapped to the luminal domain, but the molecular details of the interaction between BoNT/A and SV2 are unknown.
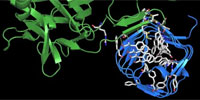
Here we determined the high-resolution crystal structure of the BoNT/A receptor-binding domain (BoNT/A-RBD) in complex with the SV2C luminal domain (SV2C-LD). SV2C-LD consists of a right-handed, quadrilateral β-helix that associates with BoNT/A-RBD mainly through backbone-to-backbone interactions at open β-strand edges, in a manner that resembles the inter-strand interactions in amyloid structures. Competition experiments identified a peptide that inhibits the formation of the complex. Our findings provide a strong platform for the development of novel antitoxin agents and for the rational design of BoNT/A variants with improved therapeutic properties.
Legend. This movie illustrates how a botulinum neurotoxin A molecule (botox) binds to its receptor. The crystal structure of the complex between the receptor binding domain of botulinum neurotoxin A (BoNT/A-RBD, shown in green) and the luminal domain of its protein receptor synaptic vesicle glycoprotein 2 (SV2C-LD, shown in blue) reveals molecular details how the toxin actually binds to SV2C-LD. The stick models in the magnification represent the amino acids at the toxin-receptor interface. The dotted lines show hydrogen bonds. The amino acids, which appear later in the movie on the inner side of SV2C-LD, are phenylalanines which form the inner core of the receptor domain.
Press releases: