Kah Wai Yau, Sam F.B. van Beuningen, Inês Cunha-Ferreira, Bas M.C. Cloin, Eljo Y. van Battum, Lena Will, Philipp Schätzle, Roderick P. Tas, Jaap van Krugten, Eugene A. Katrukha, Kai Jiang, Phebe S. Wulf, Marina Mikhaylova, Martin Harterink, R. Jeroen Pasterkamp, Anna Akhmanova, Lukas C. Kapitein, and Casper C. Hoogenraad
http://dx.doi.org/10.1016/j.neuron.2014.04.019
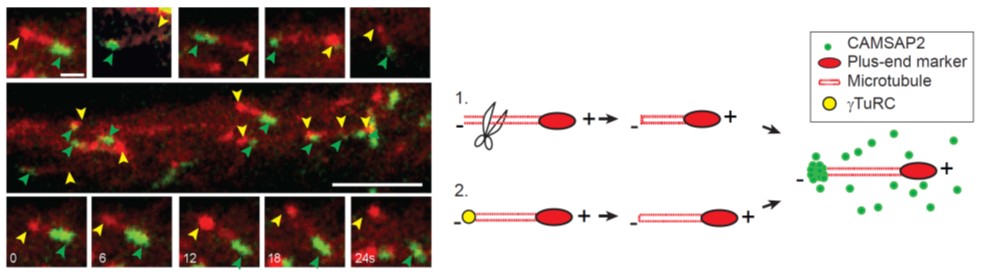
In neurons, most microtubules are not associated with a central microtubule-organizing center (MTOC), and therefore, both the minus and plus-ends of these noncentrosomal microtubules are found throughout the cell. Microtubule plus-ends are well established as dynamic regulatory sites in numerous processes, but the role of microtubule minus-ends has remained poorly understood. Using live-cell imaging, high-resolution microscopy, and laser-based microsurgery techniques, we show that the CAMSAP/Nezha/Patronin family protein CAMSAP2 specifically localizes to noncentrosomal microtubule minus-ends and is required for proper microtubule organization in neurons. CAMSAP2 stabilizes noncentrosomal microtubules and is required for neuronal polarity, axon specification, and dendritic branch formation in vitro and in vivo. Furthermore, we found that noncentrosomal microtubules in dendrites are largely generated by γ-Tubulin dependent nucleation. We propose a two-step model in which γ-Tubulin initiates the formation of noncentrosomal microtubules and CAMSAP2 stabilizes the free microtubule minus-ends in order to control neuronal polarity and development.