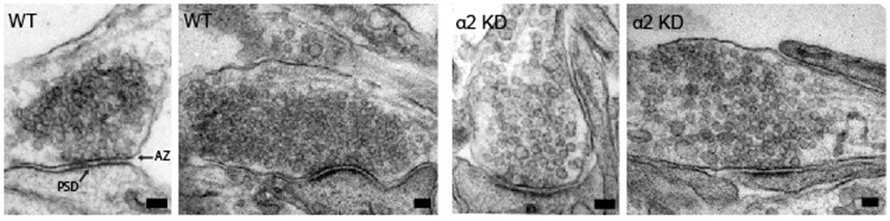
Samantha A. Spangler, Sabine K. Schmitz, Josta T. Kevenaar, Esther de Graaff, Heidi de Wit, Jeroen Demmers, Ruud F. Toonen and Casper C. Hoogenraad. Liprin-α2 promotes the presynaptic recruitment and turnover of RIM1/CASK to facilitate synaptic transmission. The Journal of Cell Biology, Vol. 201, No. 6, 10 June 2013.
Nerve cells communicate via specialized contact sites called synapses by the controlled release of neurotransmitter containing synaptic vesicles. Accurate regulation of this vesicle release at the presynaptic site is essential to ensure proper information processing and its deregulation is often associated with neurological disorders. Modulation of the molecular composition of the presynaptic active zone is important for many types of synaptic plasticity. We identify the synaptic scaffold protein liprin-α2 as a key organizer in this process. We show that liprin-α2 organizes presynaptic ultrastructure and controls synaptic output by regulating the number of releasable synaptic vesicles. The presence of liprin-α2 at presynaptic sites does not depend on other active zone scaffolding proteins but is critical for recruitment of several components of the synaptic vesicle release machinery, including RIM1 and CASK. Depletion of liprin-α2 results in reduced dynamics of RIM1 and CASK at presynaptic terminals, suggesting that liprin-α2 provides dynamic scaffolding for molecular complexes that facilitate synaptic vesicle release. Moreover, liprin-α2 levels are regulated by synaptic activity and the ubiquitin-proteasome system providing plasticity in response to changes in network activity. Together, our study demonstrates that liprin-α2 plays an important role in controlling presynaptic composition and dynamics to modulate synaptic efficacy in response to changes in network activity.






 Anna Akhmanova:
Cellular Dynamics
Anna Akhmanova:
Cellular Dynamics Lukas Kapitein: Biophysics
Lukas Kapitein: Biophysics Florian Berger:
Theoretical Biophysics
Florian Berger:
Theoretical Biophysics Agathe Chaigne:
Cell division dynamics
Agathe Chaigne:
Cell division dynamics Ginny Farías:
Neuronal Organelle Dynamics
Ginny Farías:
Neuronal Organelle Dynamics Martin Harterink:
C. elegans neurobiology
Martin Harterink:
C. elegans neurobiology Casper Hoogenraad: Molecular Neuroscience
Casper Hoogenraad: Molecular Neuroscience Maarten Kole:
Axonal Signalling
Maarten Kole:
Axonal Signalling Harold MacGillavry:
Synapse organization
Harold MacGillavry:
Synapse organization Sabrina Oliveira:
Molecular Targeted Therapies
Sabrina Oliveira:
Molecular Targeted Therapies Ihor Smal: Image Analysis, Smart Microscopy and AI
Ihor Smal: Image Analysis, Smart Microscopy and AI Frederik Verweij:
Extracellular Vesicle Biology
Frederik Verweij:
Extracellular Vesicle Biology